TIL Therapy Breakthrough: New Hope for Solid Tumor Treatment in 2025
Introduction
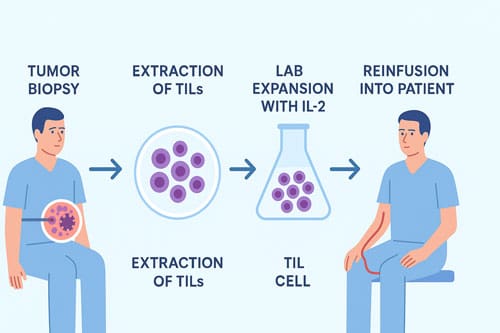
Tumor-Infiltrating Lymphocytes (TILs) represent a groundbreaking advancement in solid cancer treatment, offering new hope to patients with remarkable response rates and extended survival outcomes. This innovative immunotherapy approach extracts immune cells directly from a patient’s tumor tissue, expands them in laboratory conditions, and then reinfuses them back into the patient to target and eliminate cancer cells. The clinical impact of TIL therapy has been substantial, with a recent FDA-approved study of lifileucel showing that 31.5% of melanoma patients experienced tumor reduction. In comparison, 43.5% maintained remission for more than twelve months.
The evolution of TIL therapy marks a pivotal shift in oncological treatment strategies. Specifically, TIL therapy has demonstrated efficacy in treating melanomas resistant to checkpoint inhibition. This critical achievement validates its potential as a life-saving intervention for patients with advanced solid tumors. A comprehensive meta-analysis encompassing 411 melanoma patients revealed an overall response rate of 41%, with complete responses observed in 12% of cases. Furthermore, when combined with immune checkpoint inhibitors such as pembrolizumab, TIL therapy has shown promising results beyond melanoma. In fact, nearly 24% of patients with various gastrointestinal tumors experienced substantial tumor reduction when treated with this combination approach. As research continues to expand, clinical trials are actively exploring applications of TIL therapy beyond melanoma, potentially transforming treatment paradigms across multiple solid tumor types.
Understanding TIL Therapy and Its Role in Solid Tumors
Tumor-infiltrating lymphocyte (TIL) therapy harnesses the patient’s immune cells that have already penetrated tumor tissue to create a personalized treatment approach for cancer. This therapeutic modality relies on the natural ability of specific T cells to recognize and attack cancer cells within the tumor microenvironment.
What is TILs therapy, and how does it differ from CAR-T
TIL therapy begins with surgically removing a portion of the patient’s tumor, from which T cells that have naturally infiltrated the tumor are isolated. These extracted TILs are then cultivated in laboratory conditions with interleukin-2 (IL-2), allowing them to multiply into billions over several weeks. Before reinfusion, patients undergo lymphodepleting chemotherapy to create space for the expanded TILs, followed by IL-2 administration to stimulate further TIL growth and activation after reinfusion.
Unlike CAR-T cell therapy, TIL therapy utilizes immune cells already primed to recognize cancer without genetic modification. The primary differences between these approaches include:
- Source of cells: CAR-T uses T cells collected from peripheral blood, whereas TIL therapy uses T cells extracted directly from tumor tissue.
- Cell engineering: CAR-T cells are genetically modified to express chimeric antigen receptors that target specific surface proteins, while TILs remain unmodified.
- Target recognition: CAR-T cells recognize a single target antigen, whereas TILs possess multiple T-cell receptor (TCR) clones capable of recognizing diverse tumor antigens.
- Solid tumor efficacy: CAR-T therapy has shown limited success against solid tumors, while TIL therapy demonstrates better clinical efficacy for these cancers, especially those with high mutation loads.
Mechanism of action: TILs targeting tumor neoantigens
The effectiveness of TIL therapy stems from these cells’ ability to recognize neoantigens—abnormal proteins resulting from somatic mutations in tumor cells. These neoantigens distinguish cancer cells from healthy tissue, providing ideal targets for immune recognition. TILs that have infiltrated tumors have already demonstrated their capacity to identify these cancer-specific markers and navigate through the tumor stroma.
During the TIL manufacturing process, researchers can identify and select TILs that best recognize patient-specific tumor antigens. This selection process involves co-culturing TILs with antigen-presenting cells containing mutated epitopes identified through whole-exome sequencing of the tumor. TILs that recognize these neoantigens are identified through cytokine secretion assays, selected, expanded, and then reinfused.
Why TILs are promising for solid tumors
TIL therapy offers several advantages for treating solid tumors compared to other immunotherapeutic approaches. First, TILs have already demonstrated their ability to infiltrate tumors, overcoming the physical barriers that often limit other immune-based therapies. Additionally, their polyclonal nature allows recognition of multiple tumor antigens simultaneously, addressing tumor heterogeneity more effectively than single-target therapies.
Moreover, TIL therapy presents minimal risk of off-target effects since these cells have undergone natural selection against self-reactivity during development. This safety profile, coupled with the demonstrated efficacy in treating melanoma, has sparked interest in expanding TIL therapy to various solid tumors, including lung, cervical, head and neck, breast, and gastrointestinal cancers.
Recent clinical outcomes further validate TIL therapy’s potential for solid tumors. In a pivotal study leading to FDA approval, 31.5% of melanoma patients experienced tumor reduction with lifileucel, and 43.5% remained in remission for over a year. Beyond melanoma, early trials have shown promising results in other solid malignancies, with response rates of 30-40% reported in heavily pretreated melanoma patients.
Milestones in TIL Therapy: From Discovery to FDA Approval
The journey of tumor-infiltrating lymphocytes (TILs) from laboratory concept to FDA-approved therapy spans over three decades, marking one of the most persistent pursuits in cancer immunotherapy history.
Early trials by Rosenberg and the NCI
The foundational work on TIL therapy began in the early 1980s at the National Cancer Institute (NCI). In 1986, Steven Rosenberg and his Team first demonstrated effective TIL therapy in murine tumors after optimizing a process for culturing TILs from tumor fragments. This preclinical success led to the first human clinical trial in 1988, where 20 patients with metastatic melanoma received their own expanded TILs alongside interleukin-2 (IL-2). The results were promising—60% of treatment-naïve patients achieved objective responses, albeit with significant IL-2-related toxicities.
Throughout subsequent decades, Rosenberg’s group at the NCI refined its approach, establishing the importance of lymphodepleting chemotherapy before TIL infusions to enhance clinical outcomes. Their research illuminated how transferred T cells expanded dramatically in patients receiving lymphodepletion, sometimes demonstrating remarkable long-term persistence. Indeed, these early studies revealed that most anti-tumor activity came from T cells recognizing mutation-derived neoepitopes—abnormal peptides presented on cancer cell surfaces.
FDA approval of lifileucel (Amtagvi) for melanoma in 2024
After years of persistent research, February 16, 2024, marked a historic milestone—the FDA granted accelerated approval to lifileucel (Amtagvi), making it the first cellular therapy approved for a solid tumor. This Iovance Biotherapeutics product received approval for adult patients with unresectable or metastatic melanoma previously treated with standard therapies, including PD-1 blockers and BRAF inhibitors when applicable.
The approval was based on a single-arm trial demonstrating an objective response rate of 31.5% among 73 patients receiving the recommended dose. Remarkably, despite extensive prior treatments in this population, 43.5% of responding patients maintained their responses beyond 12 months. The therapy involves a comprehensive regimen: lymphodepleting chemotherapy with cyclophosphamide and fludarabine, followed by lifileucel infusion and up to six doses of IL-2 to support cell expansion.
Clinical response rates in melanoma and NSCLC
Beyond melanoma, TIL therapy has shown promising results in non-small cell lung cancer (NSCLC). In the Phase II multicenter trial IOV-COM-202, 21% of previously treated NSCLC patients responded to TIL therapy. Notably, one patient achieved a complete metabolic response lasting 26 months with overall disease control continuing at 30 months post-treatment.
In melanoma, longer-term data have been particularly encouraging. At the ESMO 2023 Immuno-Oncology Congress, researchers reported that among 153 heavily pre-treated patients receiving lifileucel, 31.4% showed objective responses. Even more compelling, a randomized Phase III trial comparing TILs to ipilimumab in melanoma patients who had progressed through anti-PD-1 treatment revealed a 49% response rate with TILs versus 21% with ipilimumab. Complete responses reached 20% in the TIL group, with significantly improved progression-free survival.
The first randomized Phase III TIL study, presented in September 2022, confirmed these benefits—showing a complete response rate of 20% for TILs versus 7% for ipilimumab. Consequently, this therapy now offers hope to patients who have exhausted conventional treatment options, particularly those with immunologically “hot” tumors containing abundant TILs.
Breakthroughs in TIL Manufacturing and Expansion
Manufacturing innovations have dramatically accelerated TIL therapy development in recent years, overcoming substantial technical hurdles that previously limited widespread clinical application. These advances focus on optimizing expansion protocols, addressing manufacturing challenges, and maintaining critical T cell characteristics.
Young TILs and rapid expansion protocols
The development of “Young TILs” in 2008 marked a critical turning point in TIL manufacturing efficiency. This approach rapidly expands TILs without the lengthy tumor recognition screening process, reducing manufacturing time to approximately 20 days. Young TILs exhibit superior characteristics, including higher antigen reactivity, longer telomeres, and increased CD27 and CD28 expression—all factors associated with improved proliferative capacity and persistence after infusion. Clinical implementation of Young TILs yielded impressive outcomes, with objective response rates of approximately 50% in refractory melanoma patients, ultimately facilitating broader therapeutic applications beyond melanoma.
The rapid expansion protocol (REP) traditionally required extensive labor and multiple containers. However, recent modifications incorporate more efficient stimulation techniques. For instance, combining T-cell receptor activation with 4-1BB agonistic stimulation dramatically accelerates TIL expansion, reducing the overall manufacturing cycle.
Centralized manufacturing challenges and solutions
Manufacturing challenges primarily revolve around contamination risks and resource intensiveness. Traditional open-system culture methods require extensive operator interventions, increasing contamination risks, and demanding highly skilled personnel. Moreover, final product volumes often reach 30-50 liters, necessitating specialized equipment and dedicated facilities.
To address these limitations, gas-permeable G-Rex flasks have emerged as game-changing tools. These flasks support large media volumes without compromising gas exchange, enabling 2310-fold cell expansion compared to 729-fold with traditional methods. Additionally, the WAVE bioreactor offers a closed-system alternative that produces comparable cell numbers in minimal volumes with reduced manipulation requirements. Centralized manufacturing facilities now achieve successful yield rates up to 90% of resected samples.
Reducing culture time while preserving T cell stemness
Preserving T cell stemness remains critical for clinical efficacy. Shortened culture duration correlates with longer telomere length, improved stemness, better persistence, and positive clinical outcomes. The Epi-R P2 protocol represents a breakthrough, reducing manufacturing time to less than three weeks while maintaining cellular quality.
This process yields approximately 60 billion T cells through a shortened protocol that preserves stem-like qualities. Notably, CD39 and CD69 double-negative memory-progenitor stem-like TILs strongly correlate with in vivo persistence and complete cancer regression. Further innovations include iPSC approaches, where TIL-derived induced pluripotent stem cells produce less-differentiated, tumor-specific T cells while maintaining stemness properties.
These manufacturing advancements collectively overcome logistical barriers, reduce production costs, and improve product quality—all crucial steps toward making TIL therapy accessible to more patients with various solid tumors.

Combination Therapies Enhancing TIL Efficacy
Recent clinical advances have focused on enhancing TIL therapy efficacy through strategic combinations with other immunomodulatory agents. These approaches aim to overcome immunosuppression within the tumor microenvironment and maximize therapeutic outcomes.
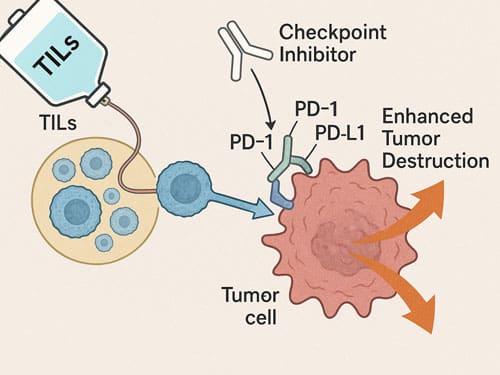
Checkpoint inhibitors like pembrolizumab with TILs
Combining TILs with immune checkpoint inhibitors has emerged as a remarkably effective strategy. When TILs were administered alongside pembrolizumab in gastrointestinal cancer patients, the objective response rate reached 23.5%, substantially higher than the 7.7% observed with TILs alone. This improvement likely occurs because pembrolizumab prevents newly introduced immune cells from becoming inactivated by the patient’s own immune system when administered immediately before TIL therapy.
The combination has proven particularly powerful in melanoma, where lifileucel plus pembrolizumab achieved an objective response rate of 65.2% with an unprecedented complete response rate of 30.4%. Beyond melanoma, this combination approach has shown promise in other malignancies, including head and neck squamous cell carcinoma (38.9% ORR) and cervical carcinoma (57.1% ORR).
IL-2 vs IL-15 vs IL-12: Cytokine co-administration strategies
Though interleukin-2 (IL-2) remains standard in TIL therapy protocols, its severe toxicity profile and ability to stimulate regulatory T cells limit its therapeutic index. Accordingly, alternative cytokines have been explored.
IL-15 offers distinct advantages over IL-2, effectively stimulating NK cells and T cells without expanding immunosuppressive Treg populations. In clinical trials, IL-15 demonstrated robust expansion of NK cells and memory CD8+ T cells, though maximum efficacy may require improved delivery methods. Alternatively, IL-12 represents another promising adjunct, particularly when tethered onto the surface of adoptively transferred cells to minimize systemic toxicity. This approach has shown increased T cell engraftment 4.7-fold compared to systemic IL-12 administration.
Neoantigen selection and personalized TILs
Recent innovations focus on selecting TILs that recognize multiple specific proteins within tumors (neoantigens), thereby increasing response rates. Methods like the NeoExpand protocol selectively expand neoantigen-reactive TILs while preserving their stem-like memory phenotypes. This approach has proven superior to conventional OKT3-induced expansion, broadening the neoantigen-reactive T cell repertoire and enabling identification of 16 unique reactivities compared to 9 with traditional methods.
Researchers are now developing sophisticated methods to identify TILs recognizing multiple tumor-specific neoantigens, creating truly personalized products. This neoantigenic stimulation approach has shown improved anti-tumor efficacy in xenograft mouse models of p53-mutated or KRAS-mutated tumors.
Expanding TIL Therapy Beyond Melanoma
TIL therapy is rapidly expanding beyond melanoma into multiple solid tumor types, showing exciting clinical activity across various malignancies with distinct biological characteristics.
TILs in gastrointestinal cancers: colon, rectal, pancreatic
Initially considered resistant to immunotherapy, gastrointestinal tumors now show promising responses to TIL therapy. In a breakthrough clinical trial with 91 patients with metastatic gastrointestinal cancers, the combination of selected TILs with pembrolizumab achieved tumor reduction in 23.5% of patients, compared to 7.7% with TILs alone. Response durations ranged from 8 months to over 5.8 years. Case reports describe remarkable outcomes, including a patient with pancreatic ductal adenocarcinoma who experienced 44.1% tumor shrinkage, plus another showing complete regression of liver metastases.
Case studies in breast and cervical cancers
Cervical cancer presents a unique opportunity for TIL therapy due to its association with HPV infection. In a phase II study of HPV-associated cervical cancer, 28% of patients responded with two complete responses lasting beyond 4 years. Likewise, a separate trial reported an impressive 44% response rate in advanced cervical cancer. For breast cancer, despite its lower mutational burden, selective neoantigen-reactive TIL therapy produced three responses among six treated patients, including one complete response lasting 5.5 years.
Challenges in low-TMB tumors and how to overcome them
Tumors with low mutational burden pose distinct challenges for TIL therapy. Essentially, fewer neoantigens result in limited targets for immune recognition. Nevertheless, researchers overcome this through enriching for neoantigen-specific TILs. As illustrated by a cholangiocarcinoma patient who experienced dramatic tumor regression after receiving CD4+ neoantigen-specific TILs, clonal selection of reactive TILs becomes necessary in lower-TMB epithelial cancers. T-cell reprogramming technologies may also help by creating rejuvenated TILs with preserved tumor-reactive properties but without exhaustion markers.

Conclusion 
TIL therapy stands as a revolutionary approach in solid tumor treatment, offering renewed hope for patients with limited therapeutic options. The recent FDA approval of lifileucel marks a watershed moment in cancer immunotherapy, validating decades of persistent research since Rosenberg’s early work at the NCI. Clinical evidence demonstrates remarkable response rates across multiple solid tumors, especially when combined with checkpoint inhibitors such as pembrolizumab.
Manufacturing breakthroughs have transformed TIL therapy from an experimental procedure into a clinically viable treatment. Young TIL protocols and rapid expansion methods have drastically reduced production times while preserving critical T cell stemness properties. These advances address previous limitations regarding scalability and accessibility.
Equally important, the polyclonal nature of TILs provides distinct advantages over single-target therapies like CAR-T. Their ability to recognize multiple tumor neoantigens simultaneously tackles tumor heterogeneity more effectively than other available approaches. This natural recognition capability explains their impressive efficacy against solid tumors that have traditionally resisted immunotherapeutic interventions.
The expansion of TIL therapy beyond melanoma represents perhaps the most promising development in this field. Encouraging results from trials in gastrointestinal, cervical, and breast cancers suggest broader applications await. Though challenges persist for tumors with low mutational burden, innovative strategies focusing on neoantigen selection and personalized approaches show potential to overcome these obstacles.
Looking ahead, TIL therapy will likely evolve through refinements in manufacturing efficiency, patient selection criteria, and combination strategies. Cytokine optimization beyond traditional IL-2 protocols may further enhance efficacy while reducing toxicity profiles. Additionally, neoantigen selection technologies will continue advancing personalized treatment approaches, maximizing therapeutic impact for individual patients.
Therefore, TIL therapy emerges not merely as another treatment option but as a fundamentally different paradigm in solid tumor management. The convergence of manufacturing innovations, combination strategies, and personalization techniques positions this approach at the forefront of next-generation cancer immunotherapies. For clinicians treating patients with refractory solid tumors, TIL therapy now offers a scientifically grounded and clinically validated alternative worth serious consideration.
Key Takeaways
TIL therapy represents a groundbreaking shift in solid tumor treatment, offering personalized immunotherapy with proven clinical success and expanding applications beyond traditional approaches.
- FDA-approved breakthrough: Lifileucel became the first cellular therapy approved for solid tumors in 2024, showing 31.5% response rates in melanoma patients, with 43.5% maintaining remission over 12 months.
- Superior solid tumor efficacy: Unlike CAR-T therapy, TILs naturally recognize multiple tumor antigens without genetic modification, making them highly effective against solid tumors that resist other immunotherapies.
- Manufacturing revolution: Young TIL protocols reduce production time to 20 days while preserving T cell stemness, making therapy more accessible and scalable for widespread clinical use.
- Powerful combination potential: TILs combined with pembrolizumab achieve remarkable 65.2% response rates in melanoma and 23.5% in gastrointestinal cancers, significantly outperforming single-agent approaches.
- Expanding beyond melanoma: Clinical trials show promising results across multiple solid tumors, including cervical (44% response rate), breast, and gastrointestinal cancers, opening new treatment possibilities for previously resistant malignancies.
This personalized approach harnesses patients’ own tumor-infiltrating immune cells, offering hope where conventional treatments have failed and establishing a new paradigm in precision cancer immunotherapy.

Frequently Asked Questions:
FAQs
Q1. What is TIL therapy, and how does it differ from other cancer treatments? TIL therapy is a personalized immunotherapy that uses a patient’s own tumor-infiltrating lymphocytes to fight cancer. Unlike CAR-T therapy, TILs are not genetically modified and can recognize multiple tumor antigens naturally, making them particularly effective against solid tumors.
Q2. What are the success rates of TIL therapy in treating melanoma? In a recent FDA-approved study, 31.5% of melanoma patients treated with lifileucel (Amtagvi) experienced tumor reduction, and 43.5% maintained remission for over 12 months. Some studies have shown even higher response rates when TILs are combined with other immunotherapies.
Q3. Can TIL therapy be used for cancers other than melanoma? Yes, TIL therapy is showing promising results in various solid tumors beyond melanoma. Clinical trials have demonstrated effectiveness in gastrointestinal cancers, cervical cancer, breast cancer, and others, with response rates ranging from 23.5% to 44% in some studies.
Q4. How long does it take to manufacture TILs for treatment? Recent advancements have significantly reduced TIL manufacturing time. The “Young TILs” approach can produce treatment-ready cells in approximately 20 days, while maintaining the cells’ effectiveness and ability to persist in the body after infusion.
Q5. Are there any strategies to enhance the effectiveness of TIL therapy? Yes, combining TIL therapy with checkpoint inhibitors like pembrolizumab has shown improved efficacy. Additionally, researchers are exploring alternative cytokines to IL-2 and developing methods to select TILs that recognize specific tumor neoantigens, further personalizing the treatment.
References:
[1] – https://pmc.ncbi.nlm.nih.gov/articles/PMC11393248/
[3] – https://jitc.bmj.com/content/11/Suppl_1/A428
[4] – https://pmc.ncbi.nlm.nih.gov/articles/PMC11082634/
[5] – https://www.astctjournal.org/article/S2666-6367(24)00800-5/fulltext
[6] – https://pmc.ncbi.nlm.nih.gov/articles/PMC7761238/
[7] – https://www.tandfonline.com/doi/full/10.1080/14712598.2023.2193290
[8] – https://pmc.ncbi.nlm.nih.gov/articles/PMC11163210/
[9] – https://www.cancer.gov/news-events/cancer-currents-blog/2024/fda-amtagvi-til-therapy-melanoma
[11] – https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/amtagvi
[13] – https://www.sciencedirect.com/science/article/pii/S2666636724007838
[14] – https://pmc.ncbi.nlm.nih.gov/articles/PMC11549075/
[15] – https://pmc.ncbi.nlm.nih.gov/articles/PMC3315105/
[16] – https://translational-medicine.biomedcentral.com/articles/10.1186/1479-5876-10-69
[17] – https://pmc.ncbi.nlm.nih.gov/articles/PMC6453723/
[18] – https://lyell.com/pdf/2023/SITC_2023_EpiR_ePoster_Final.pdf
[21] – https://www.science.org/doi/10.1126/sciadv.abi8075
[22] – https://jitc.bmj.com/content/12/5/e008645
[24] – https://www.astctjournal.org/article/S2666-6367(24)00783-8/fulltext
[25] – https://pmc.ncbi.nlm.nih.gov/articles/PMC12274268/
[26] – https://www.astctjournal.org/article/S2666-6367(24)00801-7/fulltext

