New Frontiers in Sickle Cell Disease: CRISPR-Based Cures
Abstract
Sickle cell disease (SCD) remains one of the most pressing global health challenges, with an estimated 300,000 infants born annually with the disorder and millions more living with its lifelong complications. The burden of SCD is disproportionately higher in resource-limited regions, where access to comprehensive care is often scarce and mortality rates remain unacceptably high. Despite advances in supportive care and disease-modifying therapies such as hydroxyurea and hematopoietic stem cell transplantation, curative options have been limited and largely inaccessible to most patients.
The recent approval of CRISPR-based gene editing therapies marks a historic milestone in the treatment of SCD, representing the first clinically validated use of CRISPR technology in human disease. Among these, CTX001 (Casgevy), a CRISPR-Cas9–engineered autologous hematopoietic stem cell therapy that reactivates fetal hemoglobin production through targeted BCL11A disruption, has demonstrated remarkable efficacy. Data from pivotal clinical trials show that all treated patients with transfusion-dependent β-thalassemia achieved transfusion independence, while all patients with SCD experienced resolution of vaso-occlusive crises, effectively establishing the potential for a functional cure. These outcomes have led to regulatory approvals of Casgevy in Europe, the United States, and the Middle East, heralding the advent of CRISPR-based therapeutics.[1]
However, the translation of these groundbreaking results into widespread clinical practice is challenged by barriers. The current price point of approximately $2.2 million per patient, coupled with the need for advanced infrastructure, highly specialized centers, and prolonged hospital stays, poses major limitations to equitable global access. Furthermore, questions remain regarding long-term durability of response, off-target effects, and the scalability of this highly complex therapeutic approach.
This review provides a careful evaluation of the evolving role of CRISPR-based therapies in SCD. It integrates evidence from clinical trials, mechanistic studies, and health-economic analyses to highlight both the transformative potential and the limitations of this rapidly advancing field. While CRISPR-based gene editing therapies represent a new innovation and potential cure for SCD, future progress will depend on addressing cost, accessibility, and delivery challenges.[2] [3] The development of next-generation editing platforms, optimization of delivery systems, and innovative payment models will be crucial in ensuring that the promise of CRISPR extends beyond high-resource settings to benefit the global SCD population.
Keywords: CRISPR-Cas9, sickle cell disease, gene therapy, BCL11A, fetal hemoglobin, health equity

1. Introduction
Sickle cell disease (SCD) exemplifies how precision medicine can transform the management of monogenic disorders that have historically lacked curative treatment options. SCD is a hereditary hemoglobinopathy associated with morbidity and premature mortality worldwide. Despite advances in supportive care, the therapeutic landscape has remained limited, with only two U.S. Food and Drug Administration (FDA)–approved medications, hydroxyurea and voxelotor designed to reduce disease severity. Allogeneic hematopoietic stem cell transplantation remains the only established cure, yet its availability is constrained by donor compatibility, access barriers, and the risk of serious complications such as graft-versus-host disease. Thus, curative therapy for the majority of patients with SCD has long been an unmet need.[4] [5]
The recent emergence of CRISPR-Cas9 genome editing has fundamentally shifted this paradigm, offering unprecedented opportunities for durable, potentially curative interventions. The translation of this technology from preclinical research to clinical application has been remarkably swift. In July 2019, Victoria Gray became the first individual with SCD to undergo a CRISPR-based therapy in the exa-cel clinical trial sponsored by Vertex Pharmaceuticals and CRISPR Therapeutics.[6] [7] This pioneering approach ultimately culminated in the approval of Casgevy, the first CRISPR-based therapy for SCD, marking a historic milestone in both gene editing and the treatment of hemoglobinopathies.
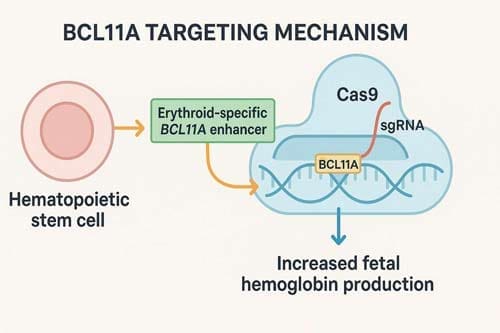
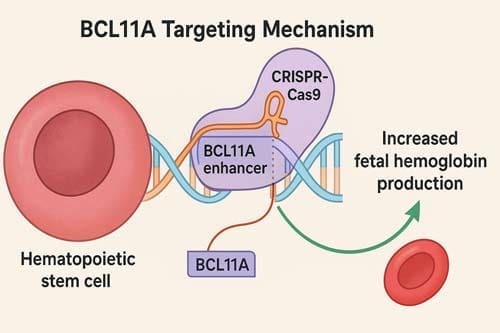
The scientific rationale for CRISPR-based interventions in SCD builds on decades of understanding of globin biology and the protective role of fetal hemoglobin (HbF). Elevated HbF levels mitigate sickling, reduce hemolysis, and protect against vaso-occlusive complications. Current CRISPR strategies harness this protective effect by editing CD34+ hematopoietic stem and progenitor cells ex vivo, with a primary approach being the targeted disruption of the BCL11A erythroid enhancer.[8] This silencing of BCL11A, a key repressor of HbF production, reactivates fetal hemoglobin synthesis, thereby compensating for the defective β-globin chains and mitigating disease manifestations. Importantly, this approach circumvents the need for direct correction of the sickle mutation, instead leveraging endogenous pathways that confer natural disease protection.
Beyond BCL11A targeting, multiple CRISPR-based strategies are being actively explored. These include direct correction of the sickle β-globin mutation through homology-directed repair, as well as emerging modalities such as base editing and prime editing, which offer enhanced precision and potentially fewer off-target effects. Each strategy presents unique advantages and challenges in terms of efficiency, safety, and scalability. The diversity of approaches underscores both the therapeutic potential of genome editing and the technical complexities of applying these innovations to clinical practice.
Ongoing clinical trials and early real-world experience provide encouraging data on the efficacy of CRISPR-based therapies in inducing sustained HbF production, reducing vaso-occlusive crises, and improving overall quality of life for patients with SCD. However, key challenges remain. These include the need for myeloablative conditioning regimens, long-term monitoring for genotoxicity and off-target effects, high treatment costs, and the ethical and logistical considerations of ensuring equitable global access to these transformative therapies.[9]
This analysis provides an in-depth overview of the current state and future prospects of CRISPR-based therapy for SCD. By examining clinical outcomes, mechanistic innovations, safety considerations, and translational barriers, we highlight how these advances are reshaping the therapeutic landscape. While major challenges remain, CRISPR-based gene editing represents a landmark in the pursuit of curative therapy for sickle cell disease and serves as a model for the broader application of precision medicine in genetic disorders.
2. Pathophysiological Foundations and Therapeutic Rationale
2.1 Molecular Basis of Sickle Cell Disease
Sickle cell disease is a hereditary hemoglobinopathy resulting from a β-globin chain mutation that causes abnormal hemoglobin (HbS) polymerization and leads to severe complications [10] [11]. The fundamental pathophysiology stems from a single nucleotide substitution in the β-globin gene, replacing glutamic acid with valine at position 6 of the β-globin chain. This seemingly minor change has profound consequences for red blood cell morphology and function.
Sickle hemoglobin polymerizes under hypoxic conditions, producing deformed red cells that hemolyze and cause vaso-occlusion that results in progressive organ damage and early death [12]. The polymerization process is complex and involves multiple factors including oxygen tension, pH, temperature, and cellular dehydration. Understanding these mechanisms has been crucial for developing targeted therapeutic interventions.
The clinical manifestations of SCD are diverse and severe, ranging from acute vaso-occlusive crises to chronic organ damage. Patients experience recurrent episodes of severe pain, acute chest syndrome, stroke, and progressive organ dysfunction affecting the spleen, kidneys, lungs, and other vital organs. SCD impacts millions and imposes severe healthcare challenges, particularly in resource-limited regions, with current treatments having variable efficacy and requiring lifelong adherence [13].
2.2 Fetal Hemoglobin as a Therapeutic Target
The therapeutic rationale for CRISPR-based approaches largely centers on the reactivation of fetal hemoglobin (HbF) production. Fetal hemoglobin reactivation expression through CRISPR-Cas9 is a promising strategy for the treatment of sickle cell disease [14] [15]. This approach is based on the well-established clinical observation that individuals with naturally elevated HbF levels experience milder disease courses.
In untreated adults, the gene product of BCL11A suppresses expression of the HBF gene, while healthy adults express normal α- and β-globin chains, leading to production of HbA and healthy erythrocytes [16] [17]. The developmental switch from fetal to adult hemoglobin production is tightly regulated by transcriptional networks involving multiple regulatory factors, with BCL11A serving as a vital repressor of γ-globin expression.
BCL11A is a transcription factor that represses γ-globin expression and fetal hemoglobin in erythroid cells, making it an ideal target for CRISPR-Cas9 targeting of the BCL11A erythroid-specific enhancer [18]. The identification of BCL11A’s role in HbF regulation represented a major breakthrough, providing a specific molecular target for therapeutic intervention.
2.3 Hereditary Persistence of Fetal Hemoglobin
Natural genetic variations that maintain elevated HbF levels into adulthood provide compelling proof-of-concept for therapeutic approaches. HPFH mutations are either deletional, where large deletions remove the δ- and β-globin genes leading to (δβ)⁰-thalassemia, or nondeletional, consisting of point mutations or small indels in the γ-globin promoters [19]. These naturally occurring mutations demonstrate that sustained HbF expression is compatible with normal erythropoiesis and can provide substantial clinical benefit.
Genome editing using CRISPR-Cas9 to create the HPFH genotype in HSPCs represents an approach for treating sickle cell disease and β-thalassemia [20] [21]. By mimicking these beneficial natural mutations, CRISPR-based therapies can potentially provide sustained therapeutic benefit without the need for ongoing medical management.
3. CRISPR Technology and Mechanistic Approaches
3.1 Fundamental CRISPR-Cas9 Technology
The CRISPR-Cas9 system represents a revolutionary advancement in genome editing technology, derived from bacterial adaptive immune systems. In 2020, Emmanuelle Charpentier and Jennifer Doudna were awarded the Nobel Prize in Chemistry for their research on the CRISPR-Cas9 method for DNA editing [22] [23]. This recognition underscores the transformative impact of CRISPR technology across multiple scientific disciplines.
Rapid and substantial progress in genome editing approaches have proven valuable as a curative option, with CRISPR/Cas9 revolutionizing genome engineering and bringing the possibility of translating these concepts into clinically meaningful reality [24]. The programmable nature of CRISPR-Cas9 allows for precise targeting of specific genomic sequences, making it ideally suited for correcting or modulating disease-causing genetic variations.
The basic mechanism involves guide RNAs that direct the Cas9 nuclease to specific DNA sequences, where it creates double-strand breaks. These breaks are then repaired by cellular mechanisms, either through non-homologous end joining (NHEJ) or homology-directed repair (HDR). The choice of repair mechanism can be influenced by experimental conditions and determines the type of genetic modification achieved.
3.2 BCL11A-Targeted Approaches
The most clinically advanced CRISPR strategy for SCD involves targeting the BCL11A gene and its regulatory elements. BCL11A is a key transcription factor that suppresses the production of fetal hemoglobin in red blood cells, and HbF upregulation could ameliorate anemia and reduce clinical complications through ex vivo CRISPR-Cas9-based gene-editing of the erythroid enhancer region of BCL11A in hematopoietic stem and progenitor cells [25] [26].
In treatment with Casgevy, SPY101 in conjunction with Cas9 causes a double-stranded break in the BCL11A promoter region, and although subsequently ‘repaired’ by inherent mechanisms, the induced indels serve to downregulate BCL11A [27]. This approach is elegant in its simplicity – by disrupting the enhancer region that drives BCL11A expression specifically in erythroid cells, the therapy effectively removes the brake on fetal hemoglobin production.
The specificity of this approach is crucial for safety considerations. BCL11A contains an erythroid-specific enhancer that is only active in RBCs, making it an ideal target for gene therapy, as interventions can be focused on erythroid cells without affecting other tissues [28]. This tissue-specific targeting minimizes the risk of off-target effects in non-hematopoietic tissues where BCL11A may have important functions.
3.3 Direct Gene Correction Strategies
An alternative approach involves directly correcting the sickle mutation in the β-globin gene. Correction of the disease causing sickle mutation using gene-editing represents the most straightforward therapeutic approach, where CRISPR gRNA/Cas9 RNP complex targeting HBB together with DNA donor template are delivered into HSPCs, resulting in HDR mediated correction of the causative mutation [29] [30].
While conceptually appealing, direct gene correction faces major technical challenges. Given that NHEJ is the favored mechanism for repairing DSBs in HSCs, this presents a major challenge for gene correction approaches, which rely on HDR [31]. The efficiency of HDR in primary hematopoietic stem cells is typically low, requiring optimization of delivery methods and repair templates.
Recent advances in base editing and prime editing technologies offer promising alternatives for direct gene correction. A custom adenine base editor (ABE8e-NRCH) was used to convert the SCD allele (HBBS) into Makassar β-globin (HBBG), a non-pathogenic variant [32]. These newer editing technologies can achieve precise nucleotide changes without requiring double-strand breaks, potentially improving safety and efficiency.
3.4 Alternative Targeting Strategies
Beyond BCL11A, researchers have identified other potential targets for HbF induction. The major fetal hemoglobin gene repressors, BCL11A and Leukemia/lymphoma-related factor (LRF), are directly bound to the HbG promoter, with CRISPR/Cas9 mediated disruption of either the LRF- or BCL11A-binding site inducing HbF production [33].
CRISPR-Cas9 disruption of the HBG1 and HBG2 gene promoters was an effective strategy for induction of fetal hemoglobin, with targeted disruption increasing fetal hemoglobin expression in red-cell progeny [34] [35] [36]. This approach directly targets the γ-globin gene promoters rather than their regulatory factors, offering an alternative mechanism for HbF induction.
4. Clinical Development and Regulatory Approval
4.1 Casgevy (CTX001): The First Approved CRISPR Therapy
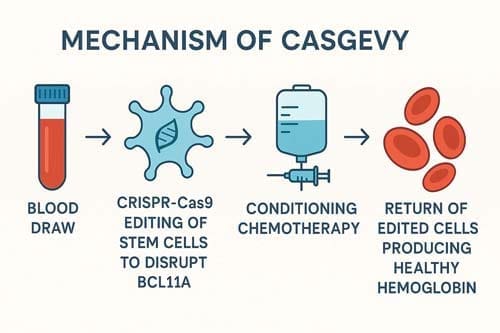
The development of Casgevy represents a landmark achievement in gene therapy and regulatory approval processes. The UK MHRA was the first to approve CRISPR-Cas9 gene editing therapy Casgevy for treatment of patients with transfusion-dependent β-thalassemia and sickle cell disease, followed by US FDA approval and European Medicines Agency approval [37].
Exagamglogene autotemcel (Casgevy™) is a genetically modified autologous CD34+ cell enriched population containing human haematopoietic stem and progenitor cells edited ex vivo by CRISPR/Cas9 to differentiate into erythroid cells that produce high levels of foetal hemoglobin, developed by Vertex Pharmaceuticals and CRISPR Therapeutics [38].
The clinical development program involved multiple phases of testing, with impressive results demonstrating both safety and efficacy. All patients demonstrated increases in total Hb and HbF over time, with TDT patients ceasing pRBC transfusions and remaining transfusion-free for over 15 months, while SCD patients had no VOCs since CTX001 infusion, with the first SCD patient remaining free of VOCs for over 1 year [39] [40].
4.2 Clinical Trial Results and Long-term Outcomes
The clinical data supporting Casgevy’s approval demonstrate sustained therapeutic benefit across multiple outcome measures. After undergoing myeloablation, two patients received autologous CD34+ cells edited with CRISPR-Cas9 targeting the BCL11A enhancer, and more than a year later, both patients had high levels of allelic editing, increases in fetal hemoglobin, transfusion independence, and elimination of vaso-occlusive episodes [41].
In preclinical experiments, CD34+ HSPCs edited with CRISPR-Cas9 had sustained on-target editing with no off-target mutations and produced high levels of fetal hemoglobin, while in the clinical study, all participants had engraftment and stable induction of fetal hemoglobin with broadly distributed F cells [42] [43].
The durability of clinical responses represents a major advantage of gene editing approaches. Clinical trials have shown that Casgevy administered to patients aged 12 years and older enables precise modifications in hematopoietic stem cells, resulting in elevated fetal hemoglobin levels and marked reduction in vaso-occlusive events, offering a curative approach that eliminates the need for recurrent transfusions and transplants [44].
4.3 Safety Profile and Adverse Events
The safety profile of CRISPR-based therapies has been generally favorable, though vigilant monitoring continues. The safety profile after CTX001 infusion was generally consistent with busulfan myeloablation, with four serious adverse events related to CTX001 reported in one patient including headache, haemophagocytic lymphohistiocytosis, acute respiratory distress syndrome, and idiopathic pneumonia syndrome, all of which were resolved or clinically improving [45].
Approximately 80% of the alleles were modified with no evidence of off-target editing, and after myeloablation, patients received autologous CD34+ cells edited with CRISPR-Cas9 targeting the BCL11A enhancer [46]. The absence of detectable off-target editing represents an important safety milestone, though continued long-term monitoring remains essential.
Recent studies have identified some disease-specific considerations for safety assessments. SCD HSPCs showed reduced engraftment and myeloid bias compared with healthy donor cells, with off-target activity and chromosomal rearrangements detected, particularly in SCD samples, though these did not impact target gene expression and HSPC engraftment and differentiation [47].
5. Alternative CRISPR Strategies and Next-Generation Approaches
5.1 Base Editing and Prime Editing Technologies
The evolution of genome editing technologies has produced increasingly sophisticated tools for precise genetic modifications. Techniques such as base editing and prime editing can correct the pathogenic variant into a non-pathogenic or wild-type one or augment fetal haemoglobin production, with optimization of these tools for efficacious gene editing with minimal off-target effects [48].
Prime editing systems can directly repair the SCD mutation in hematopoietic stem cells in vivo, with a single intravenous injection resulting in correction of ∼40% of βS alleles in HSCs and replacement of 43% of sickle hemoglobin with adult hemoglobin [49] [50] [51]. This in vivo approach represents an impressive advancement over ex vivo editing protocols, potentially reducing complexity and cost.
The advantages of newer editing technologies include reduced dependence on double-strand break repair and improved precision. Custom optimization is needed for delivering epigenome/base/prime editors, with promising results shown in numerous pre-clinical studies and clinical trials, though safe and effective delivery methods remain a key challenge for in vivo genome editing therapy [52] [53].
5.2 Epigenome Editing Approaches
CRISPR-based epigenome editing represents an emerging frontier that could complement or supersede traditional gene editing approaches. CRISPR/Cas-based epigenome editing allows site-specific control over modifications to DNA, histones, and chromatin architecture, with patient-derived induced pluripotent stem cells and epigenome editing permitting precision disease modeling [54].
The repurposing of prokaryotic CRISPR systems has allowed for the development of diverse technologies for epigenome engineering, with current achievable epigenetic manipulations and corresponding applications representing powerful tools for understanding and controlling biological function [55].
These approaches could potentially provide more nuanced control over gene expression patterns, allowing for fine-tuning of therapeutic outcomes. The reversible nature of epigenetic modifications may also offer advantages in terms of safety and adjustability of therapeutic effects.
5.3 In Vivo Delivery Systems
The development of effective in vivo delivery systems represents a key frontier for expanding access to gene editing therapies. The discovery of CRISPR genome editing technology opened the door to provide a versatile approach for treating multiple diseases, with promising results in numerous pre-clinical studies and clinical trials, though safe and effective delivery methods remain a key challenge [56].
In vivo CRISPR therapeutics require potent and safe delivery vectors, with CRISPR-based genome editing advancing rapidly from primary research to clinical trials, though the lack of safe and potent in vivo delivery methods has limited most ongoing clinical trials to ex vivo gene therapy [57] [58].
Multiple vector systems are under development, including viral and non-viral approaches. AAV is one of the most widely used vectors for in vivo CRISPR-Cas delivery, though immunogenicity against capsid, liver toxicity at high dose, and potential genotoxicity remain unsolved, while VLP and LNP are emerging technologies for transient CRISPR-Cas9 and gRNA delivery [59] [60] [61].
6. Economic Considerations and Access Challenges
6.1 Cost-Effectiveness Analysis
The economic implications of CRISPR-based SCD therapies present both opportunities and challenges for health systems worldwide. Gene therapy for SCD below a $2 million price tag is likely to be cost-effective when applying a societal perspective at an equity-informed threshold for cost-effectiveness analysis [62] [63]. This analysis suggests that despite high upfront costs, the long-term value proposition may be favorable.
Gene therapy for severe sickle cell disease is likely to produce considerable budget impact for many Medicaid plans while potentially offering substantial benefit to patients, with payers needing to take steps to ensure affordability and access, likely providing an early demonstration of unique financial challenges associated with emerging drug classes [64].
The cost-effectiveness calculations must consider multiple factors including prevented hospitalizations, reduced transfusion requirements, improved quality of life, and long-term complications avoided. Using GRACE-adjusted incremental cost-effectiveness ratio estimates, both lovo-cel and exa-cel therapies would be considered cost-effective, with implementing the GRACE framework resulting in 6% reduction in incremental cost-effectiveness ratios and willingness-to-pay thresholds increased by approximately 50% [65] [66].
6.2 Pricing and Reimbursement Challenges
The pricing of CRISPR-based therapies has generated conflicting debate regarding affordability and access. Casgevy, the world’s first approved CRISPR-based cell therapy, has been priced at $2.2 million per patient, and although this hefty price tag was widely anticipated, the extremely high cost poses a major ethical issue in terms of equitable access and global health [67].
The large eligible population size – by far, the largest for a gene therapy – poses daunting budget challenges and threatens to exacerbate health disparities for Black patients, who make up the vast majority of American sickle cell patients [68] [69]. This concern highlights the intersection of clinical innovation with health equity considerations.
Despite clinical successes, the high costs and complex manufacturing and delivery requirements present major challenges to broad use and equitable access, with disparities ranging from financial constraints to infrastructure limitations and regulatory hurdles requiring innovative strategies including alternative manufacturing models and innovative payment structures [70].
6.3 Global Health Implications
The global burden of SCD is disproportionately concentrated in resource-limited settings, creating particular challenges for implementing high-cost therapies. Although ex vivo gene-editing has many advantages including high editing efficiency, the high cost will prevent applicability to patients with SCD in resource-poor regions, with attempts being made to develop in vivo HSC transduction/selection technology [71].
The development of more accessible delivery methods and manufacturing approaches will be essential for global implementation. Potential solutions to lower the prices of CRISPR gene therapies must be developed, as the approval of CRISPR transforms our obligations of justice and compels us to bring future gene therapies to the maximum possible number of patients [72].
7. Safety Considerations and Long-term Monitoring
7.1 Genotoxicity and Off-Target Effects
The safety profile of CRISPR-based therapies requires careful evaluation of potential genotoxic effects and off-target editing. Off-target activity and chromosomal rearrangements were detected, particularly in SCD samples likely due to higher overall editing efficiency, but did not impact target gene expression and HSPC engraftment and differentiation, though transcriptomic analyses showed up-regulation of genes involved in DNA damage and inflammatory responses [73].
To translate gene-editing based strategies to the clinic, many challenges exist, including potential off-target effects, the need to increase efficiency of gene correction, and in vivo engraftment of gene-edited HSPCs, with optimization of genome-editing methods being critical for achieving high safety and efficacy [74] [75].
The development of more precise editing tools and improved delivery methods continues to address these safety concerns. Recent technological advances have focused on reducing off-target effects through improved guide RNA design, enhanced Cas9 variants with reduced off-target activity, and better delivery systems that minimize exposure to non-target tissues.
7.2 Long-term Monitoring Requirements
The long-term safety of gene editing therapies requires comprehensive monitoring protocols to detect potential delayed effects. Casgevy represents a first-in-class therapeutic with numerous considerations including risk of tumorigenicity and off-target editing, limited cohort size, validity of proposed dosing, and single-arm studies, though the sponsors’ claims were considered well supported by their data [76].
The regulatory approval of Casgevy included requirements for long-term follow-up studies to monitor for potential delayed effects including malignancy risk, chromosomal instability, and durability of therapeutic effects. These studies will provide crucial data for understanding the long-term benefit-risk profile of CRISPR-based therapies.
7.3 Disease-Specific Safety Considerations
Recent research has identified disease-specific factors that may influence safety outcomes in SCD patients. Transcriptomic analyses showed that the editing procedure results in up-regulation of genes involved in DNA damage and inflammatory responses, which was more evident in SCD HSPCs, highlighting the need for performing safety studies in clinically relevant conditions using patient-derived HSPCs [77].
These findings suggest that safety assessments conducted in healthy donor cells may not fully capture the risk profile in SCD patients, emphasizing the importance of disease-specific safety studies and monitoring protocols.
8. Future Directions and Emerging Technologies
8.1 Next-Generation Editing Tools
The rapid evolution of genome editing technologies continues to produce new tools with improved precision and reduced toxicity. The growing understanding of CRISPR biology and its application to genome, epigenome and transcriptome engineering is narrowing the gap between sequencing and editing capabilities, with various CRISPR-based systems able to transiently or permanently modify the genome and transcriptome [78].
Emerging technologies including prime editing, base editing, and epigenome editing offer potential advantages over traditional CRISPR-Cas9 approaches. These newer tools may provide more precise control over genetic modifications while reducing the risk of unintended consequences associated with double-strand break formation.
8.2 Improved Delivery Systems
The development of more efficient and safer delivery systems represents a critical area for future advancement. Within less than a decade, CRISPR-Cas9-based genome editing has rapidly advanced to human clinical trials, though the low efficiency of in vivo delivery must be enhanced before therapeutic potential can be fully realized, with recent progress highlighting innovative viral and non-viral delivery technologies.
Biological vectors offer easy mammalian cell production and efficient transduction, with mammalian-based delivery vehicles being developed as less immunogenic vectors and engineering of biological-derived vectors generating next-generation platforms [79].
8.3 Combination Approaches and Personalized Medicine
Future therapeutic strategies may involve combining multiple approaches to optimize outcomes for individual patients. Gene therapy advancements include gene addition via lentiviral vectors and gene editing with CRISPR/Cas9 technology, with clinical trials bringing notable progress though challenges remain including leukemogenesis, delivery efficiency and cost, requiring future efforts to focus on enhancing efficiency, reducing costs, and developing nongenotoxic conditioning regimens.
The integration of pharmacogenomic data, disease severity assessments, and individual risk profiles may enable more personalized treatment approaches that optimize therapeutic benefit while minimizing risks and costs.

9. Regulatory and Ethical Considerations
9.1 Regulatory Framework Evolution
The approval of CRISPR-based therapies has established important precedents for regulatory evaluation of gene editing technologies. Ethical considerations surrounding CRISPR technology and regulatory frameworks must be addressed to ensure responsible application and equitable access to this one-time gene editing therapy, with sustained interdisciplinary collaboration and ethical scrutiny essential for navigating the evolving landscape [80].
The regulatory pathways established for Casgevy will likely influence the development and approval of future gene editing therapies, establishing standards for efficacy demonstration, safety monitoring, and risk-benefit assessment.
9.2 Ethical Implications of Gene Editing
The clinical implementation of CRISPR-based therapies raises important ethical questions regarding access, equity, and the appropriate use of genetic modification technologies. Ethical considerations surrounding CRISPR technology and regulatory frameworks must be addressed to ensure responsible application and equitable access, with sustained interdisciplinary collaboration and ethical scrutiny essential to navigating the evolving landscape [81].
Key ethical considerations include ensuring equitable access across different populations, managing the risks and benefits of genetic modification, and establishing appropriate consent processes for permanent genetic changes.
9.3 Global Implementation Challenges
The global implementation of CRISPR-based SCD therapies faces challenges related to infrastructure, expertise, and resource allocation. The development of sustainable approaches for delivering these therapies in resource-limited settings will require innovative solutions including technology transfer, capacity building, and alternative funding mechanisms.
Discussion and Critical Analysis
The emergence of CRISPR-based therapies for SCD represents a paradigm shift in treating hereditary diseases, offering unprecedented opportunities for curative interventions. The clinical success of Casgevy demonstrates the feasibility of genome editing approaches, with sustained clinical benefits observed across multiple outcome measures. However, several critical challenges must be addressed to realize the full potential of these technologies.
The mechanistic approach of targeting BCL11A enhancer regions represents an elegant solution that leverages natural biology to achieve therapeutic benefit. These early data demonstrate that CTX001 is a potential functional cure for the treatment of TDT and SCD [82]. The durability of responses and the magnitude of clinical benefit support the transformative potential of this approach.
However, significant limitations remain in translating these advances to broader patient populations. The complexity of manufacturing, the need for specialized infrastructure, and the high costs associated with current approaches limit accessibility. Lowering the prices of future CRISPR therapies is an urgent ethical imperative [83] that requires coordinated efforts across multiple stakeholders.
The safety profile of current approaches appears favorable, though long-term monitoring remains essential. Safety studies in clinically relevant conditions using patient-derived HSPCs highlight the need for disease-specific safety assessments [84]. The observation of enhanced editing efficiency in SCD cells compared to healthy donor cells underscores the importance of conducting safety studies in relevant disease contexts.
Future developments in delivery systems, editing tools, and manufacturing processes may address current limitations. Future efforts must focus on enhancing efficiency, reducing costs, developing nongenotoxic conditioning regimens and methods for in vivo application. The development of in vivo delivery approaches could potentially revolutionize the field by eliminating the need for ex vivo cell manipulation and myeloablative conditioning.
The economic considerations surrounding CRISPR-based therapies require careful balancing of innovation incentives with accessibility concerns. While cost-effectiveness analyses support the value proposition of these therapies, the upfront costs create key setbacks to implementation, particularly for health systems serving predominantly disadvantaged populations.
Conclusions and Future Perspectives
CRISPR-based gene editing has ushered in a new era of curative treatments for sickle cell disease, with the approval of Casgevy marking a historic milestone in both gene therapy and SCD management. The clinical evidence demonstrates sustained therapeutic benefit with favorable safety profiles, supporting the transformative potential of genome editing approaches.
The success of BCL11A-targeted strategies validates the therapeutic rationale and establishes a foundation for further innovations. The diversity of emerging approaches, including base editing, prime editing, and epigenome editing, suggests continued evolution of the field with potentially improved precision and reduced complexity.
However, significant challenges remain in achieving equitable global access to these transformative therapies. The high costs, complex manufacturing requirements, and need for specialized infrastructure create obstacles that must be addressed through coordinated efforts involving industry, regulators, payers, and global health organizations.
Future research priorities should focus on developing more accessible delivery methods, reducing manufacturing costs, improving editing precision, and establishing sustainable implementation models for resource-limited settings. The integration of next-generation editing tools with improved delivery systems may enable more efficient and cost-effective approaches.
The regulatory precedents established through Casgevy’s approval will influence the development pathway for future gene editing therapies. Continued refinement of regulatory frameworks, safety monitoring protocols, and efficacy standards will be essential for supporting innovation while protecting patient safety.
The ethical implications of gene editing technologies require ongoing attention, particularly regarding equitable access and appropriate use of genetic modification approaches. The development of global governance frameworks and ethical guidelines will be important for ensuring responsible implementation of these powerful technologies.
In conclusion, CRISPR-based approaches have fundamentally transformed the therapeutic landscape for SCD, offering unprecedented opportunities for curative interventions. While significant challenges remain in achieving broad implementation, the continued evolution of editing technologies, delivery systems, and implementation strategies provides reason for optimism about the future of gene editing in SCD treatment and beyond.
The journey from laboratory discovery to clinical implementation has been remarkably rapid, demonstrating the potential for scientific innovation to address long-standing medical challenges. As the field continues to evolve, the lessons learned from SCD applications will inform broader efforts to develop gene editing therapies for other hereditary diseases, potentially revolutionizing the treatment of genetic disorders worldwide.
The success of CRISPR-based SCD therapies represents not only a medical breakthrough but also a proof-of-concept for the broader potential of genome editing in precision medicine. As we continue to refine these approaches and address implementation challenges, the promise of curative genetic treatments for millions of patients worldwide moves closer to reality.
References:
[1] Five Years of Progress in CRISPR Clinical Trials (2019-2024) – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/39436279/
[2] Exagamglogene Autotemcel: First Approval – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/38228954/
[3] Exagamglogene Autotemcel: First Approval – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/38228954/
[4] CRISPR/Cas9 for Sickle Cell Disease: Applications, Future Possibilities, and Challenges – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/30715679/
[5] CRISPR/Cas9 for Sickle Cell Disease: Applications, Future Possibilities, and Challenges – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/30715679/
[6] Five Years of Progress in CRISPR Clinical Trials (2019-2024) – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/39436279/
[7] Five Years of Progress in CRISPR Clinical Trials (2019-2024) – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/39436279/
[8] CRISPR-Cas9 Editing of the HBG1 and HBG2 Promoters to Treat Sickle Cell Disease – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/37646679/
[9] In vivo HSC prime editing rescues sickle cell disease in a mouse model – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S0006497123004317
[10] Safety and efficacy studies of CRISPR-Cas9 treatment of sickle cell disease highlights disease-specific responses – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/39044427/
[11] Safety and efficacy studies of CRISPR-Cas9 treatment of sickle cell disease highlights disease-specific responses – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/39044427/
[12] CRISPR-Cas9 Editing of the HBG1 and HBG2 Promoters to Treat Sickle Cell Disease – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/37646679/
[13] Gene therapy for sickle cell disease: recent advances, clinical trials and future directions – ScienceDirect –https://www.sciencedirect.com/science/article/abs/pii/S1465324924009253
[14] Safety and efficacy studies of CRISPR-Cas9 treatment of sickle cell disease highlights disease-specific responses – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/39044427/
[15] Safety and efficacy studies of CRISPR-Cas9 treatment of sickle cell disease highlights disease-specific responses – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/39044427/
[16] Revolutionising healing: Gene Editing’s breakthrough against sickle cell disease – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S0268960X24000183
[17] Revolutionising healing: Gene Editing’s breakthrough against sickle cell disease – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S0268960X24000183
[18] Revolutionary breakthrough: FDA approves CASGEVY, the first CRISPR/Cas9 gene therapy for sickle cell disease – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/39118728/
[19] Gene therapy for sickle cell disease: recent advances, clinical trials and future directions – ScienceDirect –https://www.sciencedirect.com/science/article/abs/pii/S1465324924009253
[20] CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/33283989/
[21] CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/33283989/
[22] Revolutionising healing: Gene Editing’s breakthrough against sickle cell disease – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S0268960X24000183
[23] Revolutionising healing: Gene Editing’s breakthrough against sickle cell disease – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S0268960X24000183
[24] CRISPR/Cas9 for Sickle Cell Disease: Applications, Future Possibilities, and Challenges – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/30715679/
[25] Exagamglogene Autotemcel: First Approval – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/38228954/
[26] Exagamglogene Autotemcel: First Approval – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/38228954/
[27] Revolutionising healing: Gene Editing’s breakthrough against sickle cell disease – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S0268960X24000183
[28] Gene therapy for sickle cell disease: recent advances, clinical trials and future directions – ScienceDirect –https://www.sciencedirect.com/science/article/abs/pii/S1465324924009253
[29] CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/33283989/
[30] CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/33283989/
[31] Gene therapy for sickle cell disease: recent advances, clinical trials and future directions – ScienceDirect –https://www.sciencedirect.com/science/article/abs/pii/S1465324924009253
[32] Revolutionising healing: Gene Editing’s breakthrough against sickle cell disease – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S0268960X24000183
[33] Gene Therapy Versus Common Care for Eligible Individuals With Sickle Cell Disease in the United States : A Cost-Effectiveness Analysis – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/38252942/
[34] CRISPR-Cas9 Editing of the HBG1 and HBG2 Promoters to Treat Sickle Cell Disease – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/37646679/
[35] CRISPR-Cas9 Editing of the HBG1 and HBG2 Promoters to Treat Sickle Cell Disease – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/37646679/
[36] CRISPR-Cas9 Editing of the HBG1 and HBG2 Promoters to Treat Sickle Cell Disease – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/37646679/
[37] Revolutionising healing: Gene Editing’s breakthrough against sickle cell disease – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S0268960X24000183
[38] FDA approval of Casgevy and Lyfgenia: a dual breakthrough in gene therapies for sickle cell disease – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/39239058/
[39] Exagamglogene Autotemcel: First Approval – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/38228954/
[40] Exagamglogene Autotemcel: First Approval – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/38228954/
[41] CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/33283989/
[42] CRISPR-Cas9 Editing of the HBG1 and HBG2 Promoters to Treat Sickle Cell Disease – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/37646679/
[43] CRISPR-Cas9 Editing of the HBG1 and HBG2 Promoters to Treat Sickle Cell Disease – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/37646679/
[44] Safety and efficacy studies of CRISPR-Cas9 treatment of sickle cell disease highlights disease-specific responses – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/39044427/
[45] Exagamglogene Autotemcel: First Approval – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/38228954/
[46] CRISPR/Cas9 for Sickle Cell Disease: Applications, Future Possibilities, and Challenges – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/30715679/
[47] CRISPR/Cas9 gene editing for curing sickle cell disease – ScienceDirect –https://www.sciencedirect.com/science/article/abs/pii/S1473050221000100
[48] In vivo HSC prime editing rescues sickle cell disease in a mouse model – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S0006497123004317
[49] In vivo somatic cell base editing and prime editing – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S1525001621004573
[50] In vivo somatic cell base editing and prime editing – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S1525001621004573
[51] In vivo somatic cell base editing and prime editing – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S1525001621004573
[52] CRISPR/Cas9 for Sickle Cell Disease: Applications, Future Possibilities, and Challenges – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/30715679/
[53] CRISPR/Cas9 for Sickle Cell Disease: Applications, Future Possibilities, and Challenges – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/30715679/
[54] CRISPR technologies for genome, epigenome and transcriptome editing – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/38308006/
[55] CRISPR/Cas9 Gene-Edited Hematopoietic Stem Cell Therapy for Sickle Cell Disease – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S0006497119810510
[56] Safety and efficacy studies of CRISPR-Cas9 treatment of sickle cell disease highlights disease-specific responses – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/39044427/
[57] In vivo delivery of CRISPR-Cas9 therapeutics: Progress and challenges – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S2211383521001866
[58] In vivo delivery of CRISPR-Cas9 therapeutics: Progress and challenges – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S2211383521001866
[59] Safety and efficacy studies of CRISPR-Cas9 treatment of sickle cell disease highlights disease-specific responses – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/39044427/
[60] Safety and efficacy studies of CRISPR-Cas9 treatment of sickle cell disease highlights disease-specific responses – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/39044427/
[61] Safety and efficacy studies of CRISPR-Cas9 treatment of sickle cell disease highlights disease-specific responses – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/39044427/
[62] Affordable Pricing of CRISPR Treatments is a Pressing Ethical Imperative – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/39392045/
[63] Affordable Pricing of CRISPR Treatments is a Pressing Ethical Imperative – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/39392045/
[64] CRISPR/Cas9 for Sickle Cell Disease: Applications, Future Possibilities, and Challenges – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/30715679/
[65] Revolutionising healing: Gene Editing’s breakthrough against sickle cell disease – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S0268960X24000183
[66] Revolutionising healing: Gene Editing’s breakthrough against sickle cell disease – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S0268960X24000183
[67] Delivery of CRISPR-Cas tools for in vivo genome editing therapy: Trends and challenges – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S016836592200027X
[68] CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/33283989/
[69] CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/33283989/
[70] Revolutionary breakthrough: FDA approves CASGEVY, the first CRISPR/Cas9 gene therapy for sickle cell disease – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/39118728/
[71] CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/33283989/
[72] Delivery of CRISPR-Cas tools for in vivo genome editing therapy: Trends and challenges – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S016836592200027X
[73] CRISPR/Cas9 gene editing for curing sickle cell disease – ScienceDirect –https://www.sciencedirect.com/science/article/abs/pii/S1473050221000100
[74] CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/33283989/
[75] CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/33283989/
[76] Revolutionising healing: Gene Editing’s breakthrough against sickle cell disease – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S0268960X24000183
[77] CRISPR/Cas9 gene editing for curing sickle cell disease – ScienceDirect –https://www.sciencedirect.com/science/article/abs/pii/S1473050221000100
[78] CRISPR/Cas9 gene editing for curing sickle cell disease – ScienceDirect –https://www.sciencedirect.com/science/article/abs/pii/S1473050221000100
[79] In vivo delivery of CRISPR-Cas9 therapeutics: Progress and challenges – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S2211383521001866
[80] Safety and efficacy studies of CRISPR-Cas9 treatment of sickle cell disease highlights disease-specific responses – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/39044427/
[81] Next-generation biological vector platforms for in vivo delivery of genome editing agents – ScienceDirect –https://www.sciencedirect.com/science/article/abs/pii/S0958166923001507
[82] Exagamglogene Autotemcel: First Approval – PubMed – pubmed.ncbi.nlm.nih.gov https://pubmed.ncbi.nlm.nih.gov/38228954/
[83] Delivery of CRISPR-Cas tools for in vivo genome editing therapy: Trends and challenges – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S016836592200027X
[84] CRISPR/Cas9 gene editing for curing sickle cell disease – ScienceDirect –https://www.sciencedirect.com/science/article/abs/pii/S1473050221000100

