Breaking Through: Latest Advances in Targeting IL-13 and IL-4 for Atopic Diseases
Introduction
Atopic dermatitis affects up to 25% of children and approximately 10% of adults, including 1-3% of the elderly, making targeting IL-13 and IL-4 in atopic diseases a critical focus for modern therapeutic development. This common inflammatory skin condition arises from a complex interaction of immune dysregulation, epidermal barrier impairment, and IgE sensitization, shaped by genetic predisposition and environmental factors. The immune hallmark of atopic dermatitis centers on type 2 inflammation, characterized by activation of T helper 2 cells, T cytotoxic 2 cells, innate lymphoid cells, γ/δ T cells, eosinophils, and mast cells.
Recent advances in understanding IL-4 and IL-13 in atopic dermatitis have revolutionized treatment approaches. Dupilumab, a fully human monoclonal antibody that blocks the interleukin (IL)-4 and IL-13 cytokines by targeting IL-4Rα, became the first AD-specific biologic approved for both adults and adolescents. This IL-13 inhibitor not only provides an effective treatment option for moderate-to-severe atopic dermatitis but also offers valuable insights into disease pathogenesis. Clinical studies demonstrate dupilumab’s robust efficacy over placebo, with tissue investigations confirming that clinical improvements correlate with reversal of molecular and epidermal changes in treated patients. Furthermore, this IL-4 inhibitor drug significantly reduces levels of key biomarkers, including thymus-regulated chemokine and activation, eotaxin-3, total immunoglobulin E, and periostin. By blocking IL-4 and IL-13 in atopic dermatitis, dupilumab helps restore the skin barrier by reducing the production of specific chemokines and cytokines, thereby decreasing inflammatory cell infiltration in the skin.
IL-4 and IL-13 Signaling Pathways in Atopic Diseases
The molecular mechanisms through which IL-4 and IL-13 exert their effects in atopic diseases rely on a sophisticated signaling network. Understanding these pathways reveals why targeting IL-13 and IL-4 in atopic diseases has emerged as a powerful therapeutic strategy. Both cytokines activate overlapping yet distinct signaling cascades that ultimately drive the pathogenic features of atopic dermatitis.
IL-4Rα and IL-13Rα1 Receptor Complexes
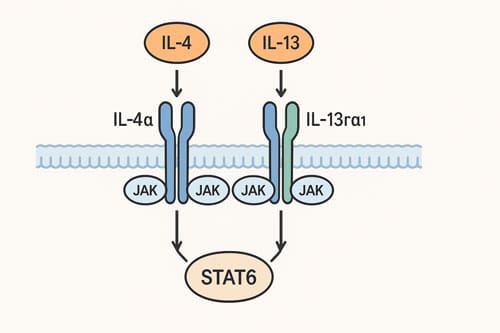
The IL-4/IL-13 receptor family consists of two distinct heterodimeric receptors with varying cellular distributions. The type I IL-4 receptor comprises IL-4Rα and the common gamma chain (γc), while the type II receptor contains IL-4Rα paired with IL-13Rα1. Although IL-4 can signal through both receptor types, IL-13 exclusively utilizes the type II complex. This receptor distribution explains the cell-specific responses to these cytokines in atopic conditions.
Hematopoietic cells predominantly express type I receptors due to higher levels of IL-4Rα and γc, consequently responding more robustly to IL-4. Conversely, non-hematopoietic cells (including keratinocytes and fibroblasts) express greater amounts of IL-13Rα1 and limited γc, making them particularly responsive to IL-13. This differential expression pattern explains why IL-13 has pronounced effects on structural cells in atopic dermatitis lesions.
Additionally, IL-13 can bind to IL-13Rα2, initially considered only a decoy receptor due to its short cytoplasmic tail (17 amino acids compared to IL-13Rα1’s 60 amino acids). However, recent evidence suggests IL-13Rα2 may activate alternative signaling pathways through interaction with chitinase-like protein (YKL-40), forming a multimeric complex capable of activating MAPK, AKT, and Wnt/β-catenin pathways.
JAK-STAT6 Activation Cascade
When IL-4 binds to IL-4Rα (with high affinity, KD = 0.1 nM), it facilitates recruitment of either γc (type I) or IL-13Rα1 (type II). This assembly activates Janus kinases (JAKs) that are constitutively associated with receptor chains: JAK1 with IL-4Rα, JAK3 with γc, and TYK2 or JAK2 with IL-13Rα1.
Following receptor engagement, these activated JAKs phosphorylate five tyrosine residues (Y497, Y575, Y603, Y631, and Y713) within IL-4Rα’s cytoplasmic domain. The phosphorylated Y575, Y603, and Y631 residues serve as docking sites for STAT6, which subsequently becomes phosphorylated at its C-terminal tyrosine residue (Y631). This phosphorylation enables STAT6 homodimerization, nuclear translocation, and binding to specific DNA motifs (TTC-N4-GAA) in STAT6-responsive genes.
In contrast to the shared STAT6 activation, only type I receptor signaling efficiently activates insulin receptor substrate-2 (IRS-2) through the phosphorylated Y497 residue of IL-4Rα. Because IL-13 signals exclusively through type II receptors, it cannot efficiently activate IRS-2-dependent pathways that lead to PI3K/AKT/mTOR activation.
Shared and Distinct Pathways of IL-4 and IL-13
Despite their overlapping receptor utilization, IL-4 and IL-13 exhibit important functional differences in atopic diseases. IL-4 primarily orchestrates the central immune response by promoting Th2 cell differentiation, IgE class switching in B cells, and the early stages of type 2 inflammation. On the other hand, IL-13 functions more peripherally at the tissue level during the effector phase of the immune response.
In atopic dermatitis pathogenesis, IL-13 dominates RNA sequencing profiles of lesional skin. Through type II receptor signaling, IL-13 directly induces epidermal hyperplasia, a hallmark of atopic dermatitis. Furthermore, IL-13 distinctively activates TNF-α expression exclusively through type II receptor signaling, whereas immune cell infiltration involves synergistic actions of both cytokines: IL-13 via type II receptors regulates T cell and macrophage accumulation, while IL-4 via type I receptors mediates eosinophil recruitment.
Apart from canonical JAK-STAT6 signaling, both cytokines can activate alternative pathways. IL-13 can trigger PI3K/AKT/PKC cascades independent of IRS-2, contributing to the expression of tenascin-C, a glycoprotein elevated in asthmatic airways. Moreover, both cytokines activate MAPK family members (ERK1/2, JNK, p38) that induce expression of inflammatory genes through transcription factors like NFAT, CREB, and AP-1.
Immune Cell Modulation by IL-4 and IL-13
IL-4 and IL-13 function as master regulators of immune responses in atopic diseases, orchestrating a complex network of cellular interactions that drive disease pathogenesis. These cytokines exert profound effects on multiple immune cell types through distinct yet overlapping mechanisms, making them prime targets for therapeutic intervention.
Th2 Differentiation via IL-4
IL-4 serves as the principal cytokine directing the differentiation of naïve CD4+ T cells into T helper 2 (Th2) cells, a process central to initiating atopic responses. Unlike IL-13, IL-4 possesses this unique ability to establish type 2 inflammation through its action on the T cell compartment. This differentiation process depends on STAT6 and GATA binding protein 3 (GATA3) transcription factors that orchestrate Th2-specific gene expression programs. Through type I IL-4 receptor signaling (IL-4Rα/γc), IL-4 not only promotes Th2 cell generation but also induces the development of T cytotoxic (Tc) 2 cells and group 2 innate lymphoid cells (ILC2).
The timing of IL-4’s influence appears critical in atopic dermatitis pathogenesis. Analysis of CD4+ cord blood T cells revealed that elevated IL-4 levels (coupled with decreased IFN-γ) correlated with increased risk for developing atopic dermatitis. Indeed, IL-4 is thought to play a key pathogenic role in the early stages of disease progression, setting the stage for subsequent inflammatory cascades. Transgenic mice overexpressing IL-4 in the epidermis developed all hallmarks of atopic dermatitis, including pruritus, increased inflammatory cell infiltration, bacterial skin infection, and elevated IgG1 and IgE levels.
IL-13-Induced Eosinophil Recruitment
In contrast to IL-4’s primary role in immune cell differentiation, IL-13 acts predominantly during the effector phase of immune responses, with potent effects on tissue cells and eosinophil recruitment. Nevertheless, both cytokines contribute to eosinophilic inflammation through distinct pathways.
IL-13 drives eosinophil trafficking to tissues primarily through induction of eotaxin family chemokines (CCL11 and CCL24). Both fibroblasts and myeloid cells produce these chemokines, notably Ly6C+ classical monocytes, in response to IL-13 stimulation. Essentially, IL-13 regulates the distribution of eosinophils within tissues, as demonstrated in studies where eotaxin-deficient mice showed normal bronchoalveolar lavage fluid eosinophilia but markedly impaired tissue eosinophilia following IL-13 exposure.
The relationship between IL-13 and IL-5 in eosinophil recruitment is particularly noteworthy. IL-5 provides an essential signal for the expansion and mobilization of eosinophils from bone marrow, whereas IL-13 mediates their tissue-specific recruitment. This cooperative mechanism is evident in studies showing that IL-13-induced airway eosinophilia was largely dependent on IL-5, with IL-5-deficient mice displaying markedly attenuated eosinophil recruitment. Conversely, IL-13 administration to IL-5 transgenic mice resulted in a 20-fold increase in eosinophils compared to wild-type mice.
B Cell Class Switching to IgE
Both IL-4 and IL-13 direct B cells to undergo immunoglobulin class switching to IgE production, a hallmark feature of atopic diseases. These cytokines deliver the first signal required for this process, inducing transcription of Cε RNA that initiates upstream of the Iε exon and proceeds through the Sε and Cε exons.
IL-4 has historically been recognized as the primary driver of this process, binding to IL-4Rα with high affinity (KD approximately 10^-10 M). This cytokine induces germ-line IgE heavy-chain gene transcription in purified B cells and promotes class switching to both IgE and IgG4 in humans (IgG1 in mice). Interestingly, in addition to its effects on B cells themselves, IL-4 enhances IgE production indirectly by upregulating CD23 expression, which further amplifies B cell responsiveness.
Though less potent than IL-4, IL-13 independently induces IgG4 and IgE synthesis through IL-4-independent mechanisms. Studies with highly purified surface IgD+ B cells demonstrated that IL-13 could induce production of these isotypes, reflecting genuine class switching rather than selective outgrowth of committed B cells. Furthermore, IL-13 plays a distinct role in IgE affinity maturation, complementing IL-4’s primary function in class-switching initiation.
When added together at optimal concentrations, IL-4 and IL-13 showed no additive or synergistic effects on IgE production, suggesting they utilize common signaling pathways in B cells. This mechanistic overlap explains why therapeutic approaches targeting both cytokines simultaneously, rather than either individually, represent particularly promising strategies for atopic disease management.
IL-4 and IL-13 in Atopic Dermatitis Pathogenesis
Structural damage to the epidermal barrier lies at the core of atopic dermatitis pathophysiology, with IL-4 and IL-13 cytokines playing decisive roles in deteriorating skin integrity, perpetuating inflammation, and triggering the itch-scratch cycle. These cytokines act through multiple mechanisms to compromise epidermal function and sensitize neural pathways, creating a self-reinforcing cycle of disease progression.
Skin Barrier Disruption via Filaggrin Downregulation
The epidermal barrier dysfunction in atopic dermatitis stems largely from IL-4 and IL-13’s ability to diminish essential structural proteins. Through STAT6 activation, both cytokines substantially decrease expression of key proteins, including filaggrin, filaggrin 2, loricrin, involucrin, keratin 1, keratin 10, hornerin, desmoglein, and desmocollin 1. This impaired protein expression, coupled with altered lipid composition (ceramides, free fatty acids, and cholesterol), fundamentally compromises normal barrier function.
The mechanistic pathway behind filaggrin downregulation involves interference with the OVOL1 signaling pathway. Specifically, IL-13 inhibits filaggrin expression by disrupting OVOL1 function, while simultaneously suppressing involucrin through an OVOL1-independent mechanism, thus exacerbating barrier dysfunction through multiple pathways. These cytokine-driven changes result in increased transepidermal water loss—a hallmark clinical feature that may even predict atopic dermatitis development.
Beyond structural protein suppression, IL-4 and IL-13 reduce production of antimicrobial peptides (AMPs) by keratinocytes. Diminished antimicrobial defense contributes to the characteristic skin dysbiosis dominated by Staphylococcus aureus colonization, which research shows typically precedes the appearance of atopic dermatitis lesions.
IL-13 in Atopic Dermatitis Lesional Skin
Even though both IL-4 and IL-13 contribute to atopic dermatitis pathogenesis, mounting evidence suggests that IL-13 predominates in lesional skin. Transcriptomic analyses reveal that IL-13 gene expression levels correlate more strongly with skin inflammation intensity than IL-4 levels. Additionally, patients with atopic dermatitis exhibit markedly higher expression of IL-13 (2.06 × 10⁻⁴) as well as IL-13RA1 (1.28 × 10⁻²) and IL-13RA2 (4.45 × 10⁻⁴) compared to healthy controls.
Upon barrier disruption, keratinocytes release alarmins—IL-33, IL-25, and TSLP—which stimulate group 2 innate lymphoid cells (ILC2s). Interestingly, these ILC2s expand and produce IL-13 and IL-5, but not IL-4, when activated by these keratinocyte-derived alarmins. It creates a self-perpetuating cycle where ‘alarmed’ keratinocytes trigger ILC2 activation, leading to increased IL-13 expression, which recruits activated T cells, resulting in amplified IL-13/IL-4 expression within the skin.
Furthermore, IL-13 contributes uniquely to the tissue remodeling observed in chronic atopic dermatitis by inducing excessive collagen production in fibroblasts and promoting fibrotic changes. This process manifests clinically as the thickened, lichenified skin lesions characteristic of chronic disease.
IL-4 and IL-13 in Pruritus Sensitization
Chronic itch represents the most burdensome symptom for atopic dermatitis patients, profoundly impacting quality of life. Recent investigations have established that both IL-4 and IL-13 function as potent pruritogenic mediators through multiple pathways.
The IL-4Rα receptor subunit—shared by both cytokines—is expressed on peripheral sensory neurons, enabling direct neuronal activation. Through this mechanism, IL-4 and IL-13 sensitize neurons to respond to subthreshold levels of various pruritogens. Calcium flux experiments confirm that a subset of neurons responds directly to IL-4 and IL-13 stimulation, dependent on extracellular calcium.
Beyond direct neural effects, IL-4 amplifies IL-31-mediated itch by enhancing the interaction between IL-31 and its receptor (IL-31Rα), leading to increased production of CCL17 and CCL22. IL-4 similarly upregulates IL-31Rα expression on sensory neurons, creating a sensitization feedback loop.
IL-13 specifically potentiates histamine-independent itch pathways through the transient receptor potential ankyrin 1 (TRPA1) channel. As a neuroactive cytokine, IL-13 increases neuronal excitability and enhances responses across multiple itch pathways. Intradermal injection studies demonstrate IL-13’s ability to induce allokinesis (itch sensitivity in surrounding skin) and direct itching sensations.
Dual vs Selective Inhibition: Mechanistic Rationale
Understanding the differential roles of IL-4 and IL-13 in atopic diseases has substantially advanced therapeutic approaches. Clinical experience reveals that inhibiting either cytokine alone yields inadequate results, whereas simultaneously blocking both produces superior outcomes. This difference stems from their distinct yet complementary functions in disease pathogenesis.
Why IL-4 Alone is Not Enough
Early therapeutic development focused separately on IL-4 or IL-13 inhibition, yielding disappointing results. Attempts at neutralizing soluble IL-4 showed limited efficacy in clinical trials. This failure occurred primarily because IL-4 inhibition alone cannot directly suppress tissue cell responses, which are essential for symptom resolution.
IL-4 certainly contributes to atopic disease pathogenesis through its central role in initiating immune responses. Yet, historical data demonstrate that anti-IL-4 agents alone consistently failed in late-phase clinical trials. Such findings align with the understanding that IL-4 predominantly affects early-stage disease processes by driving T cell differentiation. In contrast, downstream effects—particularly on tissue cells—remain relatively unaffected by IL-4 blockade alone.
Upon reviewing failed monotherapy approaches, researchers noted that selective IL-4 targeting misses critical aspects of the inflammatory cascade, especially the tissue-level inflammation persisting even with IL-4 suppression. Hence, IL-4 monotherapy generally delivers delayed therapeutic responses without addressing the effector phase of atopic inflammation.
Tissue-Specific Effects of IL-13
IL-13 exerts distinct tissue-specific effects that complement yet differ from IL-4’s actions. Unlike IL-4, which orchestrates immune cell differentiation, IL-13 primarily acts peripherally at the tissue level via type II receptors. In fact, transcriptomic analyses reveal IL-13 gene expression substantially outweighs IL-4 expression in atopic dermatitis lesional skin, underscoring IL-13’s dominant role in affected tissues.
The tissue effects of IL-13 manifest through several mechanisms. First, IL-13 directly influences keratinocytes to enhance CCL26 (eotaxin-3) production, thereby recruiting eosinophils to eczematous skin. Second, IL-13 uniquely contributes to airway remodeling and mucus hypersecretion in asthmatic patients. Third, IL-13 distinctly regulates tissue eosinophilia without affecting circulating eosinophil levels, as demonstrated in experimental models.
Of particular importance, IL-13 receptor distribution patterns determine tissue-specific responses. Non-hematopoietic structural cells express higher levels of IL-13Rα1 but limited γc, making them particularly responsive to IL-13. Throughout the disease course, IL-13 primarily influences peripheral tissue cells during the effector phase of immune responses, regulating pathogenic processes that IL-4 alone cannot effectively control.
Advantages of Dual IL-4/IL-13 Blockade
Given the distinct yet complementary roles of IL-4 and IL-13, dual inhibition offers several mechanistic advantages. First, simultaneous blockade addresses multiple phases of the inflammatory cascade—IL-4’s role in initiating immune responses and IL-13’s effects on effector tissues. This dual approach prevents both the differentiation of inflammatory cells and their tissue-specific actions.
Second, clinical evidence supports this mechanistic rationale. Dupilumab, which blocks IL-4Rα and thereby inhibits both IL-4 and IL-13 signaling, demonstrates superior efficacy to either monotherapy. This effectiveness extends across multiple atopic conditions—asthma, atopic dermatitis, nasal polyposis, and eosinophilic esophagitis—suggesting a fundamental advantage to targeting both pathways.
Third, dual blockade prevents pathogenic cross-talk between immune and tissue cells. Only combined IL-4/IL-13 blockade effectively inhibits Th2 cell-induced antigen-presenting cell activation and potently suppresses lung eosinophilia, inflammatory cytokines, and chemokine expression. Therefore, dual inhibition affects multiple cell types throughout the inflammatory cascade, breaking the self-perpetuating cycle of inflammation more effectively than targeting either cytokine alone.
The collective evidence explains why something “appears unique about targeting IL-4 and IL-13 jointly that does not manifest by targeting them individually”, establishing a clear mechanistic rationale for dual inhibition strategies in atopic disease management.
Approved IL-4/IL-13 Inhibitors and Their Mechanisms
Recent therapeutic advances have yielded several approved agents targeting IL-4 and IL-13 signaling pathways in atopic diseases. These biologics represent a paradigm shift in treatment by offering precise cytokine inhibition without broad immunosuppression, providing relief for patients with moderate-to-severe disease.
Dupilumab: IL-4Rα Blockade
Dupilumab, a fully human IgG4 monoclonal antibody, blocks both IL-4 and IL-13 signaling by binding to the shared IL-4 receptor alpha chain (IL-4Rα) present in both receptor complexes. This strategic binding site enables dupilumab to inhibit signaling through both type I (IL-4Rα/γc) and type II (IL-4Rα/IL-13Rα1) receptors simultaneously. Through this dual mechanism, dupilumab prevents downstream STAT6 activation and effectively halts the signaling cascade initiated by both cytokines.
The binding affinity of dupilumab to IL-4Rα is remarkably high, with KD values of 33pM for monomeric and 12 pM for dimeric human IL-4Rα. Following administration, dupilumab completely blocks IL-4Rα expression and STAT6 phosphorylation in CD19+ B cells and CD4+ T cells within 2 hours, with effects persisting through 52 weeks of treatment.
At the molecular level, dupilumab treatment significantly reduces the signature of more than 800 genes affected in atopic dermatitis, including Th2 chemokines, T-cell proliferation genes, and dendritic cell-related genes. The efficacy of this approach is evident in clinical trials where 64-72% of patients treated with dupilumab 300 mg weekly achieved EASI-75 compared to only 22% in placebo groups.
Pitrakinra: IL-4 Mutein Binding IL-4Rα
Pitrakinra (AER-001, BAY-16-9996) represents a distinct approach to cytokine inhibition as an IL-4 mutein—a recombinant protein derived from human IL-4. Unlike antibody-based therapies, pitrakinra functions as a competitive antagonist by binding to IL-4Rα without triggering signaling, thereby blocking the activities of both IL-4 and IL-13.
Experimentally, pitrakinra demonstrated potent inhibitory activity against IL-4/IL-13-mediated proliferative effects in vitro and effectively reduced allergen-induced inflammation in animal models of asthma and skin inflammation. A unique feature of pitrakinra is its versatility in administration routes—both subcutaneous and inhaled formulations have been tested, with superior effects observed with inhaled formulations in asthma trials.
In clinical testing, subcutaneous pitrakinra significantly reduced allergen-induced airway hyperresponsiveness, with a maximum effect of 2.8-3.8-fold increase in methacholine PC100 relative to control at b.i.d. doses of 0.05-0.5 mg/kg. Likewise, inhaled pitrakinra showed similar efficacy at nominal b.i.d. doses of 3-100 mg.
Tralokinumab and Lebrikizumab: IL-13 Specific Inhibitors
Unlike dual inhibitors, tralokinumab and lebrikizumab selectively target IL-13 without affecting IL-4 signaling. Tralokinumab, a fully human IgG4 monoclonal antibody, binds to IL-13 with high affinity and prevents its interaction with both IL-13Rα1 and IL-13Rα2 receptors. In contrast, lebrikizumab specifically binds to soluble IL-13 at an epitope that overlaps with the IL-4Rα binding site, preventing signaling through the IL-4Rα/IL-13Rα1 heterodimeric receptor while preserving IL-13’s binding to IL-13Rα2.
This mechanistic distinction explains their differing clinical profiles. Tralokinumab, approved by the FDA in early 2022 for moderate-to-severe atopic dermatitis, demonstrated efficacy in phase III trials (ECZTRA 1-3). After 16 weeks, 56% of tralokinumab-treated patients achieved EASI-75 versus 35.7% with placebo. For lebrikizumab, clinical trials showed 69.5% of patients achieved EASI-75 versus 42.2% in the placebo group.
Both selective IL-13 inhibitors exhibit favorable safety profiles with mainly mild-to-moderate adverse effects. Conjunctivitis appears as a common side effect across these therapies, occurring at higher rates than placebo in clinical trials. The efficacy of selective IL-13 inhibition provides compelling evidence that antagonizing IL-13 alone may be sufficient to control atopic dermatitis in many patients.
Comparative Efficacy in Clinical Trials
Clinical trials evaluating IL-13 and IL-4 inhibitors offer valuable comparative insights regarding efficacy, timing of response, and safety considerations for practitioners treating atopic dermatitis.
EASI-75 and IGA 0/1 Outcomes
Head-to-head evaluations reveal distinct efficacy profiles among biologic therapies. Meta-analyses show that IL-13 antagonists (lebrikizumab and tralokinumab) substantially improve EASI scores compared to placebo (mean difference -20.37, 95%CI -32.28, -8.47). In pivotal lebrikizumab trials, EASI-75 was achieved by 58.8% versus 16.2% for placebo in trial 1, and 52.1% versus 18.1% in trial 2. Tralokinumab similarly demonstrated efficacy, with 56% of patients achieving EASI-75 compared to 35.7% for placebo plus topical corticosteroids.
Upon examining IGA 0/1 outcomes, lebrikizumab-treated patients showed response rates of 43.1% versus 12.7% for placebo in trial 1, and 33.2% versus 10.8% in trial 2. Tralokinumab achieved an IGA 0/1 response in 38.9% of patients versus 26.2% for placebo.
Direct comparison between biologics and JAK inhibitors reveals interesting patterns—upadacitinib demonstrated superior EASI-75 response at week 16 compared to dupilumab (72.4% versus 62.6%). Upadacitinib also yielded better EASI-90 and EASI-100 responses (61.6% versus 40.3% and 28.4% versus 7.9%, respectively).
Onset of Action: IL-13 vs IL-4 Inhibitors
Temporal analysis indicates that JAK inhibitors typically work faster than biologics. Upadacitinib demonstrates a more rapid onset, with 44.3% of patients achieving EASI-75 at week 2 compared to 18.2% with dupilumab. IL-13 inhibitors show a time-dependent effect, with efficacy emerging around week 4.
Regarding itch relief, upadacitinib improves pruritus scores as early as week 1 (32.0% improvement versus 8.9% with dupilumab). Lebrikizumab shows dose-dependent improvements in pruritus from the first assessment at week 4.
Safety Profiles: Conjunctivitis and Eosinophilia
Safety analysis across trials reveals that IL-13 inhibition correlates with increased conjunctivitis risk (RR 2.318, 95%CI 1.471, 3.652). Interestingly, tralokinumab treatment is associated with higher conjunctivitis rates (RR 2.453, 95%CI 1.479, 4.068), yet prevalence remains similar between lebrikizumab (6.3%) and tralokinumab (6.2%).
Conjunctivitis rates vary among therapies—occurring in 8.8% of dupilumab patients versus only 1.5% with upadacitinib—rates with lebrikizumab range from 1.4% to 3.8% across dose groups.
Regarding hematologic parameters, lebrikizumab causes transient eosinophil increases at week 4 that approach baseline by week 16. In contrast, dupilumab-associated eosinophilia occurs in 4-25% of patients, occasionally persisting beyond six months. This difference may relate to dupilumab’s inhibition of IL-4/IL-13-driven eosinophil migration from circulation to tissues.
Emerging IL-4 and IL-13 Inhibitor Drugs in Development
Beyond approved therapies, several promising candidates targeting IL-4 and IL-13 pathways are advancing through clinical development pipelines. These innovative molecules offer potential improvements in efficacy, dosing convenience, and safety profiles.
CBP-201 and AK120: IL-4Rα Monoclonal Antibodies
Rademikibart (formerly CBP-201), a next-generation human IgG4 kappa monoclonal antibody, blocks IL-4Rα-mediated signal transduction for both IL-4 and IL-13 pathways. Phase I trials demonstrated its favorable safety profile with mostly mild treatment-emergent adverse events and only two injection site reactions. At week 4, patients experienced substantial improvements across multiple efficacy measures—74.4% reduction in Eczema Area and Severity Index (EASI) scores and 52.8% improvement in pruritus severity. Notably, inflammatory biomarker concentrations decreased by 55.4% in rademikibart treatment arms versus an 18.0% increase with placebo. The drug exhibited greater than dose-proportional exposure, suggesting nonlinear clearance that might enable less frequent dosing. Alongside rademikibart, AK120 represents another novel IL-4Rα monoclonal antibody currently undergoing phase II clinical evaluation.
ASLAN004 and Anrukinzumab: IL-13Rα1 Targeting
ASLAN004 (now eblasakimab) employs a distinct mechanism by targeting IL-13Rα1 rather than the cytokine itself or IL-4Rα. This fully human monoclonal antibody uniquely blocks signaling of both IL-4 and IL-13 by preventing receptor complex formation. Early trials showed complete inhibition of STAT6 phosphorylation within one hour of dosing. Remarkably, the trough level required to completely inhibit signal transduction completely appears “over an order of magnitude lower than existing therapies”. In phase 2 development, eblasakimab demonstrated 61% improvement in EASI scores versus 32% with placebo. Meanwhile, anrukinzumab (IMA-638) functions similarly to lebrikizumab by binding IL-13, yet preserves a mechanistically distinct profile.
Pascolizumab: IL-4 Neutralizing Antibody
Pascolizumab (SB 240683) represents an earlier approach focusing exclusively on IL-4 neutralization. This humanized monoclonal antibody directly binds and neutralizes IL-4, preventing its interaction with receptors. Preclinical studies demonstrated rapid IL-4 binding with slow dissociation rates. Despite promising in vitro activity inhibiting IL-4-dependent events, including IL-5 synthesis, TH2 cell activation, and IgE upregulation, clinical development stalled after phase II trials in asthma revealed inadequate efficacy despite acceptable safety. This outcome aligns with the current understanding that targeting IL-4 alone yields insufficient clinical benefit in atopic conditions.
Future Directions in IL-4 and IL-13 Targeting
Advancements in understanding IL-4 and IL-13 pathways are shifting treatment paradigms toward precision approaches for atopic diseases.
Biomarker-Guided Therapy for IL-13 in Atopic Dermatitis
Baseline lesional skin CCL22 expression emerges as the strongest predictor of clinical improvement across multiple atopic dermatitis therapies. Alongside, baseline levels of the Th17 cell-related cytokine CXCL2 demonstrate robust predictive value for dupilumab response. These findings allow objective outcome measures for better comparison between treatments. Currently, CCL17/TARC stands as the biomarker with the most supporting evidence, yet several others, including CCL22/MDC, CCL26/Eotaxin-3, and CCL27/CTACK, show promise in correlating with disease severity.
Combination Therapies with JAK Inhibitors
Real-world studies reveal potential benefits of combining dupilumab with JAK inhibitors for refractory moderate-to-severe atopic dermatitis. This approach capitalizes on complementary mechanisms—dupilumab targets IL-4Rα associated with JAK-1, whereas JAK inhibitors block numerous cytokine pathways simultaneously. Even though only 8% of patients developed mild conjunctivitis (which resolved spontaneously), the safety profile of long-term combination therapy remains unexplored.
Personalized Medicine Based on Cytokine Profiles
Genetic variations profoundly influence treatment efficacy, as evidenced by how single-nucleotide polymorphisms affect pitrakinra response. Patient stratification based on molecular endotypes offers opportunities for tailored therapeutic choices. Those classified into non-type 2 endotypes may benefit from broader-acting JAK inhibitors, considering that eosinophil-high endotypes might respond better to anti-IL5 treatments.
Conclusion 
Advances in targeting IL-13 and IL-4 have transformed therapeutic approaches for atopic diseases over the past decade. Research clearly demonstrates these cytokines function through overlapping yet distinct pathways, with IL-4 predominantly orchestrating central immune responses while IL-13 exerts powerful effects on tissue cells during the effector phase. This mechanistic understanding explains why dual inhibition strategies consistently outperform single-cytokine targeting across clinical trials.
The clinical success of dupilumab, which blocks both IL-4 and IL-13 signaling through IL-4Rα inhibition, confirms the value of simultaneously addressing multiple aspects of type 2 inflammation. Consequently, patients experience improvements in barrier function, reduced pruritus, and normalized immune responses. Though selective IL-13 inhibitors like tralokinumab and lebrikizumab also show robust efficacy, their mechanism addresses primarily tissue-level effects without fully suppressing immune cell differentiation and activation.
Several promising agents currently undergoing clinical development may further expand treatment options. Rademikibart and AK120 target IL-4Rα similarly to dupilumab but potentially offer improved pharmacokinetics. Additionally, novel approaches such as eblasakimab (ASLAN004) employ unique mechanisms by targeting IL-13Rα1, potentially offering advantages through different receptor blockade strategies.
The evolution toward biomarker-guided therapy represents another notable advancement. CCL22, CXCL2, and CCL17/TARC serve as objective outcome measures that may eventually guide treatment selection based on individual patient profiles. Combination approaches with JAK inhibitors show promise for refractory cases, albeit with safety profiles requiring further evaluation through long-term studies.
The paradigm shift toward targeted immunomodulation rather than broad immunosuppression marks a true breakthrough for patients with moderate-to-severe atopic diseases. Future research will undoubtedly refine these approaches through endotype classification, allowing practitioners to match specific therapeutic agents with individual patient characteristics. Personalized medicine based on cytokine profiles thus stands poised to become the next frontier in managing these challenging conditions, potentially transforming outcomes for millions of patients worldwide.
Key Takeaways
Recent breakthroughs in targeting IL-13 and IL-4 cytokines are revolutionizing treatment for atopic diseases, offering patients with moderate-to-severe conditions new hope through precision immunotherapy approaches.
- Dual inhibition outperforms single-target therapy: Blocking both IL-4 and IL-13 simultaneously yields superior clinical outcomes compared to targeting either cytokine alone, as these molecules work through complementary pathways.
- IL-4 drives immune initiation while IL-13 dominates tissue damage: IL-4 primarily orchestrates early immune responses and T-cell differentiation, whereas IL-13 causes the structural skin barrier disruption characteristic of atopic dermatitis.
- Dupilumab’s success validates the dual-blocking approach: This FDA-approved biologic achieves 64-72% EASI-75 response rates by simultaneously inhibiting both cytokines through IL-4Rα receptor blockade.
- Biomarker-guided therapy is emerging: CCL22 and CXCL2 levels can predict treatment response, paving the way for personalized medicine approaches based on individual cytokine profiles.
- Next-generation therapies show promise: Novel agents like rademikibart and eblasakimab offer potential improvements in dosing convenience and efficacy through innovative receptor-targeting mechanisms.
The shift from broad immunosuppression to targeted cytokine inhibition represents a paradigm change in atopic disease management, with combination therapies and personalized approaches poised to transform patient outcomes further.

Frequently Asked Questions:
FAQs
Q1. What are the latest advancements in targeting IL-13 for atopic dermatitis? Lebrikizumab is a new humanized monoclonal antibody that specifically targets IL-13. It works by inhibiting the formation of the IL-4Rα/IL-13Rα1 receptor complex while allowing IL-13 internalization through IL-13Rα2. This medication has been approved for treating moderate-to-severe atopic dermatitis in adults and adolescents who are eligible for systemic therapy.
Q2. How do IL-4 and IL-13 contribute to atopic dermatitis? IL-4 and IL-13 play crucial roles in driving type 2 inflammation, which is characteristic of atopic dermatitis. These cytokines contribute to various aspects of the disease, including skin barrier dysfunction, immune cell activation, and symptom development. Their involvement has led to the development of new therapeutic approaches targeting these cytokines and their signaling pathways.
Q3. What distinguishes IL-4 from IL-13 in their signaling mechanisms? While IL-4 can signal through both type I (IL-4Rα/γc) and type II (IL-4Rα/IL-13Rα1) receptors, IL-13 signals exclusively through the type II receptor. IL-4 binds to IL-4Rα with high affinity, initiating receptor heterodimerization with either γc or IL-13Rα1. This difference in receptor usage contributes to their distinct yet overlapping functions in atopic diseases.
Q4. How does pitrakinra work in treating asthma and eczema? Pitrakinra is an IL-4 mutein that acts as a dual antagonist for both IL-4 and IL-13. It binds to the IL-4Rα subunit, effectively blocking the inflammatory responses induced by both IL-4 and IL-13. This mechanism makes it a potential treatment option for conditions like asthma and eczema, where these cytokines play significant roles.
Q5. Why is dual inhibition of IL-4 and IL-13 considered more effective than targeting either cytokine alone? Dual inhibition of IL-4 and IL-13 has shown superior clinical outcomes compared to targeting either cytokine individually. This is because these cytokines work through complementary pathways in atopic diseases. While IL-4 primarily drives early immune responses and T-cell differentiation, IL-13 is more involved in tissue-level effects and structural changes. By blocking both cytokines, therapies can address multiple aspects of the disease process, leading to more comprehensive symptom relief and improved patient outcomes.
References:
[1] – https://onlinelibrary.wiley.com/doi/10.1111/all.14151
[2] – https://www.jacionline.org/article/S0091-6749(23)00143-4/fulltext
[3] – https://jamanetwork.com/journals/jamadermatology/fullarticle/2761466
[4] – https://pubmed.ncbi.nlm.nih.gov/21157648/
[5] – https://www.sciencedirect.com/science/article/pii/S0022202X21001524
[7] – https://pmc.ncbi.nlm.nih.gov/articles/PMC7532907/
[8] – https://pmc.ncbi.nlm.nih.gov/articles/PMC8015935/
[9] – https://www.nature.com/articles/s41467-021-22834-5
[10] – https://pmc.ncbi.nlm.nih.gov/articles/PMC7317958/
[11] – https://www.nejm.org/doi/full/10.1056/NEJMoa2206714
[13] – https://pmc.ncbi.nlm.nih.gov/articles/PMC9570949/
[14] – https://pmc.ncbi.nlm.nih.gov/articles/PMC10151557/
[15] – https://pmc.ncbi.nlm.nih.gov/articles/PMC4251801/
[16] – https://www.science.org/doi/10.1126/sciimmunol.aaw2938
[17] – https://www.sciencedirect.com/science/article/pii/S1323893020300113
[18] – https://ascpt.onlinelibrary.wiley.com/doi/10.1111/cts.13899
[19] – https://pmc.ncbi.nlm.nih.gov/articles/PMC6930967/
[20] – https://www.tandfonline.com/doi/full/10.2147/ITT.S260370
[21] – https://onlinelibrary.wiley.com/doi/10.1111/j.1398-9995.2009.02156.x
[22] – https://pmc.ncbi.nlm.nih.gov/articles/PMC9364267/
[23] – https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.
2023.1165098/full
[24] – https://www.drugsincontext.com/selective-il-13-inhibitors-for-the-treatment-of-atopic-dermatitis/
[25] – https://pmc.ncbi.nlm.nih.gov/articles/PMC11574908/
[26] – https://jamanetwork.com/journals/jamadermatology/fullarticle/2782803
[27] – https://www.jiaci.org/revistas/vol32issue3_1.pdf
[28] – https://pubmed.ncbi.nlm.nih.gov/37849431/
[29] – https://www.researchgate.net/publication/374830543_Rademikibart_CBP_-201_a_next-generation_monoclonal_antibody_targeting_human_IL-4Ra_Two_phase_I_randomized_trials_
in_healthy_individuals_and_patients_with_atopic_dermatitis
[31] – https://pmc.ncbi.nlm.nih.gov/articles/PMC1906490/
[32] – https://academic.oup.com/cei/article-abstract/130/1/93/6461203
[33] – https://www.mdpi.com/1422-0067/22/24/13655
[34] – https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/pascolizumab
[35] – https://onlinelibrary.wiley.com/doi/10.1111/all.16108
[36] – https://onlinelibrary.wiley.com/doi/full/10.1155/dth/9515524
[37] – https://pmc.ncbi.nlm.nih.gov/articles/PMC8706302/
[38] – https://pmc.ncbi.nlm.nih.gov/articles/PMC11278138/

