Hidden Signs of Subclinical Cushing’s Syndrome: New Diagnostic Criteria for 2025

Introduction
Subclinical Cushing’s syndrome (SCS) presents a striking diagnostic paradox. While the classic form of Cushing’s syndrome is rare, with an estimated prevalence of approximately 1 case per 100,000 individuals, subclinical Cushing’s has been reported in up to 79 cases per 100,000. This makes it nearly 80 times more common, yet it remains frequently overlooked in clinical practice. The gap between its prevalence and recognition highlights the need for greater clinical awareness and a nuanced understanding of its presentation.
Unlike overt Cushing’s syndrome, which is characterized by the well-known triad of central obesity, moon facies, and proximal muscle weakness, subclinical Cushing’s syndrome is defined by biochemical evidence of cortisol excess in the absence of the classic constellation of physical features. The condition often manifests subtly, with abnormalities detected in only one of the three standard diagnostic tests: the 1 mg overnight dexamethasone suppression test, late-night salivary cortisol, or 24-hour urinary free cortisol. This partial or inconsistent test positivity complicates diagnosis, creating uncertainty about when to label biochemical findings as clinically significant.
Adding to this complexity, subclinical cortisol hypersecretion often coexists with common metabolic conditions such as hypertension, type 2 diabetes mellitus, obesity, and dyslipidemia. While a strong association has been observed, the causal relationship remains debated, as these comorbidities are highly prevalent in the general population. Nevertheless, evidence suggests that even modest degrees of cortisol autonomy may contribute to increased cardiovascular risk and impaired metabolic control, underscoring the importance of timely recognition.
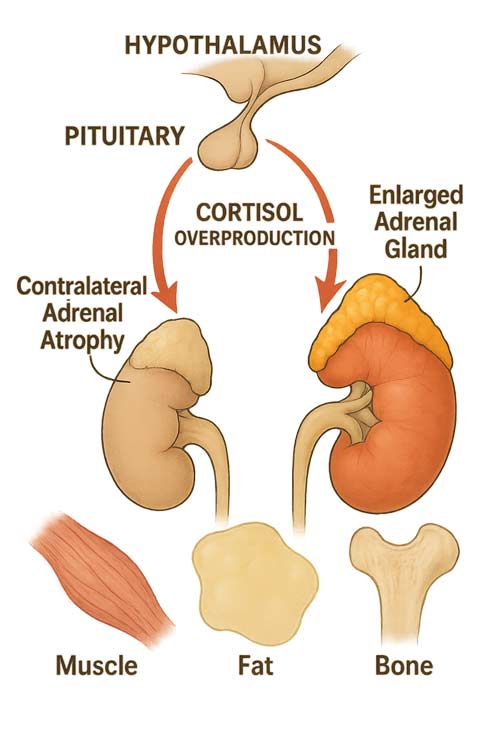
From a pathophysiological standpoint, subclinical Cushing’s reflects a spectrum of adrenal autonomy. Some patients demonstrate only mild alterations in diurnal cortisol rhythm, whereas others progress to more advanced dysfunction, including suppression of adrenocorticotropic hormone (ACTH) and contralateral adrenal atrophy. The condition is particularly relevant in the context of adrenal incidentalomas, which are increasingly identified with the widespread use of imaging. Studies suggest that between 0.2% and 7% of adults, particularly older individuals, harbor adrenal incidentalomas, and a substantial proportion of these demonstrate biochemical features consistent with subclinical cortisol excess. The prevalence of SCS rises with age, reflecting both the higher rate of incidentalomas and the cumulative effects of altered hypothalamic–pituitary–adrenal axis regulation.
In 2025, diagnostic strategies for subclinical Cushing’s continue to evolve. Updated criteria emphasize the importance of integrating biochemical findings with clinical context, particularly the presence of metabolic complications or radiological features of adrenal tumors. Treatment decisions remain individualized, balancing the potential benefits of surgical adrenalectomy against the risks of intervention, especially in asymptomatic or minimally symptomatic patients. Growing evidence indicates that surgical treatment may improve metabolic outcomes in carefully selected individuals, while others may be best managed with active surveillance and medical optimization of comorbidities.
This article explores the hidden signs, refined diagnostic approaches, and evolving treatment strategies for subclinical Cushing’s syndrome, offering clinicians practical insights into the management of this increasingly recognized endocrine disorder. With its high prevalence, noteworthy metabolic implications, and diagnostic ambiguity, subclinical Cushing’s represents a condition that demands both vigilance and discernment in modern endocrine practice.
Understanding Subclinical Cushing’s Syndrome in 2025
The diagnostic landscape of subclinical Cushing’s has evolved notably since its initial recognition. Medical understanding now encompasses a spectrum of cortisol dysregulation that extends beyond classic manifestations, necessitating updated clinical approaches for identification and management.
Definition of subclinical hypercortisolism
Subclinical hypercortisolism refers to a state of autonomous cortisol production without the full-blown clinical manifestations classically associated with Cushing’s syndrome. This condition exists on a continuum of hypothalamic-pituitary-adrenal axis dysfunction, characterized primarily by biochemical evidence of cortisol excess with minimal or absent overt physical stigmata. The 2025 consensus guidelines define subclinical Cushing’s as the presence of at least two abnormal results from the following tests: 1) elevated late-night salivary cortisol, 2) inadequate suppression after low-dose dexamethasone suppression test (LDDST), 3) suppressed morning ACTH levels, and 4) elevated 24-hour urinary free cortisol.
The pathophysiology involves varying degrees of adrenal autonomy. In the mildest cases, only subtle alterations in diurnal cortisol rhythm may be present. As the condition progresses, patients may exhibit partial resistance to dexamethasone suppression. In advanced cases, complete autonomy can result in contralateral adrenal atrophy due to chronic ACTH suppression, yet still without developing the classic cushingoid appearance.
Particularly noteworthy is the association between subclinical Cushing’s and metabolic abnormalities. Insulin resistance often precedes more obvious manifestations, subsequently increasing risk for type 2 diabetes. Likewise, hypertension, dyslipidemia, and central adiposity frequently accompany subclinical hypercortisolism, creating diagnostic challenges when differentiating cause from effect.
Prevalence in patients with adrenal incidentalomas
Adrenal incidentalomas represent the most common context for identifying subclinical Cushing’s syndrome. Current epidemiological data indicate that approximately 5-30% of patients with adrenal incidentalomas demonstrate evidence of autonomous cortisol secretion. This wide range reflects varying diagnostic criteria and testing methodologies across studies.
The prevalence of adrenal incidentalomas themselves increases with age, ranging from 0.2% in young adults to approximately 7% in older populations. Consequently, subclinical Cushing’s syndrome demonstrates a similar age-related pattern. When stratified by tumor size, larger adrenal adenomas (>3 cm) show higher rates of autonomous cortisol secretion compared to smaller lesions.
Recent radiological advances including specialized MRI sequences and functional imaging techniques now allow better characterization of these lesions. Adenomas demonstrating lipid-rich composition on chemical shift imaging correlate more frequently with subclinical cortisol production than lipid-poor lesions.
Gender distribution shows a female predominance, with women approximately twice as likely to develop subclinical Cushing’s syndrome compared to men. Additionally, familial clustering suggests potential genetic factors contributing to susceptibility, though specific genetic markers remain under investigation.
Distinction from overt Cushing’s syndrome
Differentiating subclinical from overt Cushing’s syndrome requires careful clinical assessment and laboratory interpretation. The fundamental distinction lies in the absence or minimal presence of classic cushingoid features despite biochemical evidence of cortisol excess.
Patients with overt Cushing’s syndrome typically present with proximal myopathy, wide purple striae, facial plethora, and supraclavicular fat pads – features rarely found in subclinical cases. Instead, subclinical patients may demonstrate only subtle signs like mild central obesity, borderline hypertension, or unexplained osteopenia that could easily be attributed to other common conditions.
Laboratory distinctions also exist. Overt Cushing’s syndrome generally shows consistent abnormalities across multiple tests, including pronounced elevations in 24-hour urinary free cortisol, late-night salivary cortisol, and complete resistance to dexamethasone suppression. Conversely, subclinical cases often demonstrate abnormalities in only one or two parameters, and the degree of abnormality tends to be less pronounced.
The natural history likewise differs substantially. Overt Cushing’s syndrome progresses predictably with worsening clinical manifestations if untreated. In contrast, subclinical Cushing’s syndrome may remain biochemically stable for years without clinical progression, or alternatively, some cases evolve toward overt disease – a conversion rate estimated at approximately 0.3% per year in recent longitudinal studies.
Nevertheless, both conditions share important cardiometabolic complications including insulin resistance, hypertension, and dyslipidemia, underscoring the importance of proper identification and management of even subtle cortisol excess states.

Why Subclinical Cushing’s Often Goes Undiagnosed
Despite advances in endocrine testing and increased awareness, subclinical Cushing’s syndrome remains one of the most frequently overlooked endocrine disorders. The subtle nature of this condition creates a perfect scenario for diagnostic oversight, with patients often cycling through multiple specialists before proper identification occurs.
Overlap with common conditions like obesity and diabetes
The diagnostic challenge of subclinical Cushing’s begins with its remarkable overlap with exceedingly common metabolic conditions. Patients typically present with features that could easily be attributed to lifestyle factors or aging rather than an underlying endocrinopathy.
Obesity, especially central adiposity, constitutes both a symptom of subclinical hypercortisolism and an independent condition affecting a substantial portion of the population. This creates a circular diagnostic dilemma: the patient’s weight gain might prompt investigation for metabolic causes while simultaneously masking the actual hormonal dysregulation.
Type 2 diabetes presents a particularly problematic overlap. Patients with subclinical Cushing’s often demonstrate insulin resistance and impaired glucose tolerance that appears indistinguishable from typical type 2 diabetes. However, a critical differentiating factor emerges in treatment response—these patients frequently exhibit unusual resistance to standard diabetic therapies.
Furthermore, these metabolic manifestations occur on a spectrum, with many patients presenting with:
- Mild hypertension responsive to standard medications
- Modest dyslipidemia without extreme values
- Borderline glucose intolerance or prediabetes
- Gradual weight gain attributed to aging or lifestyle
Physicians accustomed to treating these common conditions rarely consider subclinical hypercortisolism as a unifying diagnosis, especially given the relative rarity of Cushing’s syndrome in general practice.
Lack of classical Cushingoid features
Whereas overt Cushing’s syndrome produces unmistakable physical changes, subclinical Cushing’s characteristically lacks these telltale signs. The absence of purple striae, facial plethora, and pronounced buffalo hump removes the visual cues that might otherwise trigger endocrine evaluation.
Many practitioners expect to see the classic “moon face” and proximal myopathy before considering Cushing’s pathology. Yet subclinical variants specifically diverge from this expectation—cortisol production is excessive enough to cause metabolic perturbations but insufficient to generate the full phenotypic expression.
Notably, when physical manifestations do appear, they emerge so gradually that both patients and providers attribute them to normal aging processes. Subtle skin thinning, easy bruising, and mild fatigue represent early manifestations that rarely prompt specific hormonal investigations. Even when these symptoms accumulate, they rarely reach the threshold that triggers consideration of a unifying endocrine disorder.
Misinterpretation of borderline test results
Perhaps the most technical barrier to diagnosis involves the interpretation of laboratory values that often fall within “gray zones.” Unlike many endocrine disorders with clear cutoff values, subclinical Cushing’s exists on a biochemical continuum.
Standard screening tests frequently yield results that straddle the boundary between normal and abnormal. The 1mg overnight dexamethasone suppression test, considered the first-line screening tool, often produces suppression values that fall just above cutoff thresholds—prompting debate about clinical significance rather than further investigation.
Additionally, diurnal variation in cortisol secretion becomes subtly blunted rather than completely abolished. Late-night salivary cortisol may show only mild elevation on some occasions while appearing normal on others, creating inconsistent clinical pictures that confuse diagnostic algorithms.
ACTH levels typically remain detectable though inappropriately low for the corresponding cortisol levels—a nuance easily overlooked without specialist interpretation. Morning cortisol values often appear deceptively normal, further obscuring the underlying pathology.
Ultimately, the combination of nonspecific symptoms, absent classical features, and ambiguous biochemical findings creates a perfect diagnostic blind spot. Identification requires both clinical suspicion and sophisticated interpretation of subtle laboratory abnormalities—a combination that remains elusive in many clinical settings.
8 Hidden Signs of Subclinical Cushing’s Syndrome
Recognizing the subtle clinical manifestations of subclinical Cushing’s requires attentiveness to patterns that individually appear benign yet collectively suggest underlying hypercortisolism. The following eight signs represent the most revealing indicators for clinicians evaluating patients with suspected subclinical cortisol excess.
1. Mild but persistent hypertension
Patients with subclinical hypercortisolism frequently present with blood pressure elevations that persist despite adequate first-line antihypertensive therapy. The hypertension typically manifests as modest elevations (systolic 140-160 mmHg) without dramatic fluctuations. Notably, these patients often require multiple antihypertensives for adequate control. The pathophysiologic mechanism involves cortisol-induced mineralocorticoid receptor activation, enhanced vascular sensitivity to catecholamines, and increased renin substrate production—distinct from essential hypertension.
2. Unexplained weight gain with central adiposity
Central adiposity disproportionate to peripheral fat accumulation constitutes a hallmark of subclinical cortisol excess. Patients characteristically report progressive weight gain concentrated in the abdominal region despite maintaining consistent dietary habits. Unlike typical metabolic syndrome, these patients often exhibit relatively thin extremities juxtaposed against truncal obesity. The fat distribution pattern results from cortisol’s preferential effect on visceral adipocytes containing higher densities of glucocorticoid receptors.
3. Osteopenia or early-onset osteoporosis
Bone mineral density reduction without obvious risk factors warrants consideration of subclinical hypercortisolism. These patients typically present with T-scores between -1.0 and -2.5, often discovered incidentally during routine screening. Predominantly trabecular bone loss (vertebral rather than femoral) creates a distinctive pattern. Accordingly, patients might display compression fractures despite only moderately reduced bone density measurements. The underlying mechanism involves cortisol-induced inhibition of osteoblast function coupled with enhanced osteoclast activity.
4. Poorly controlled type 2 diabetes despite treatment
Insulin resistance represents one of the earliest and most consistent metabolic derangements in subclinical Cushing’s. Patients exhibit glycemic control that defies standard therapeutic approaches, requiring escalating medication regimens. Postprandial hyperglycemia often proves more prominent than fasting elevations. In fact, many patients maintain HbA1c levels 1-2% above target despite adherence to comprehensive treatment protocols. Cortisol directly antagonizes insulin action at receptor and post-receptor levels while simultaneously enhancing hepatic gluconeogenesis.
5. Fatigue and proximal muscle weakness
Subtle proximal muscle weakness, particularly in the hip girdle, indicates potential subclinical hypercortisolism. Patients typically describe difficulty rising from chairs or climbing stairs without obvious neuromuscular disease. Morning fatigue disproportionate to sleep quality represents another revealing symptom. Importantly, these manifestations lack the dramatic presentation seen in overt Cushing’s yet remarkably impact quality of life. The underlying mechanism involves cortisol-induced protein catabolism predominantly affecting type II muscle fibers.
6. Mood changes or cognitive fog
Affective disturbances including irritability, anxiety, and mild depression frequently accompany subclinical cortisol excess. Patients report cognitive inefficiency, described as “brain fog,” that fluctuates in intensity. These neuropsychiatric manifestations stem from cortisol’s effects on hippocampal function and monoamine neurotransmission. Interestingly, cognitive symptoms often improve dramatically following successful treatment, suggesting direct causality rather than coincidental occurrence.
7. Low ACTH with normal cortisol rhythm
Laboratory evaluation revealing low-normal or suppressed ACTH levels (5-15 pg/mL) alongside normal total cortisol represents a biochemical signature of subclinical autonomous adrenal function. The hallmark finding involves preservation of circadian rhythm but with higher baseline cortisol production. This pattern contrasts with both normal physiology (where ACTH appropriately drives cortisol) and overt Cushing’s (where rhythm is typically abolished).
8. Vertebral fractures without trauma
Atraumatic vertebral fractures, especially in patients younger than 65, strongly suggest underlying subclinical hypercortisolism. These fractures typically affect the thoracic spine and may present with minimal symptoms beyond progressive height loss. The pathophysiology involves preferential cortisol-induced degradation of trabecular bone combined with reduced bone formation—creating structural vulnerability even with modest reductions in bone density.
Diagnostic Testing Pathway for 2025
Accurate identification of subclinical Cushing’s syndrome requires a methodical testing approach that balances sensitivity with specificity. The diagnostic algorithm for 2025 incorporates refined cutoff values and sequential testing strategies designed to detect subtle hypothalamic-pituitary-adrenal axis abnormalities before overt clinical manifestations develop.
Low-dose dexamethasone suppression test (LDDST)
The cornerstone of subclinical Cushing’s screening remains the low-dose dexamethasone suppression test, available in two formats: overnight and two-day protocols. For the overnight test, 1 mg dexamethasone is administered at 11 PM with cortisol measurement between 8-9 AM the following morning. The two-day protocol involves 0.5 mg dexamethasone every 6 hours for 48 hours with subsequent cortisol measurement.
In 2025, the recommended cutoff value for serum cortisol stands at 1.8 μg/dL (50 nmol/L), providing approximately 95% diagnostic sensitivity. At this threshold, the two-day test demonstrates superior specificity (97-100%) compared to the overnight protocol (86%). Yet notably, even with this stringent cutoff, approximately 18% of confirmed Cushing’s disease patients show cortisol suppression below 5 μg/dL, while 8% suppress below 2 μg/dL.
Multiple factors can confound LDDST interpretation, including medications affecting CYP3A4 (phenytoin, fluoxetine, ritonavir), altered dexamethasone metabolism, estrogen therapy, and acute illness. Hence, estrogen-containing medications should ideally be discontinued at least 6 weeks before testing.
Late-night salivary cortisol collection
Late-night salivary cortisol assessment emerges as the earliest detector of abnormal cortisol rhythm in many subclinical cases. Collection involves using a cotton pledget between 11 PM-midnight, making it exceptionally convenient for outpatient screening.
For subclinical Cushing’s, a cutoff value of 0.18 μg/dL offers 82% sensitivity with 60% specificity. This test demonstrates excellent utility primarily for ACTH-dependent forms but shows lower sensitivity (31.3%) for ACTH-independent subclinical hypercortisolism.
Importantly, salivary cortisol remains stable at room temperature, allowing samples to be mailed to laboratories—a practical advantage over serum testing. Enzyme immunoassays paradoxically may outperform more analytically specific liquid chromatography-tandem mass spectrometry methods in diagnostic performance.
24-hour urinary free cortisol (UFC) limitations
Previously considered essential, 24-hour UFC measurements now play a more limited role in subclinical Cushing’s evaluation. The primary limitation stems from normal UFC values in up to 20-25% of Cushing’s syndrome patients, particularly those with mild disease.
This diminished sensitivity occurs because free cortisol only appears in urine when exceeding plasma binding capacity. A patient with consistently elevated but not extreme serum cortisol may maintain normal UFC while still experiencing tissue-level hypercortisolism effects.
Additionally, technical limitations include assay variability, complete collection challenges, and altered cortisol metabolism. Over 99% of cortisol undergoes hepatic metabolism before renal excretion, creating a substantial gap between daily cortisol production and urinary detection.
ACTH measurement and CRH stimulation test
Morning ACTH measurement represents the crucial first step in distinguishing ACTH-dependent from ACTH-independent subclinical Cushing’s. Values below 5 pg/mL strongly suggest adrenal autonomy, while levels above 15 pg/mL indicate ACTH-dependent causes.
The corticotropin-releasing hormone (CRH) stimulation test serves as a second-line investigation when initial results prove ambiguous. Following CRH administration, patients with pituitary-dependent subclinical Cushing’s typically demonstrate a >35% increase in ACTH and >20% increase in cortisol from baseline. This response pattern achieves excellent sensitivity (100%) but disappointing specificity (8%) for patients with ACTH concentrations between 5-20 pg/mL.
In 2025 diagnostic algorithms, CRH testing interpretation relies more on pattern recognition than absolute values. Blood samples are collected at -15, -1, +15, +30, +45, +60, +90, and +120 minutes relative to CRH administration, creating a response profile more diagnostically valuable than any single measurement.
When to Treat: Monitoring vs Adrenalectomy
The therapeutic approach for subclinical Cushing’s syndrome presents a clinical dilemma, balancing the risks of surgery against the benefits of resolving subtle hypercortisolism. Management decisions remain somewhat empirical given the lack of randomized prospective studies that definitively establish optimal treatment pathways.
Criteria for surgical referral in subclinical cases
Selecting appropriate candidates for surgical intervention involves careful evaluation of both biochemical parameters and clinical manifestations. Initially, adrenalectomy should be considered for patients meeting these evidence-based criteria:
- Serum cortisol ≥5 μg/dL after a 1-mg dexamethasone suppression test, particularly with treatment-resistant comorbidities (hypertension, obesity, glucose intolerance, osteoporosis, or dyslipidemia)
- Tumor diameter ≥3 cm or demonstrating growth during follow-up
- Imaging characteristics raising concern for adrenocortical carcinoma
Even as minimally invasive techniques have reduced surgical risks, the decision to operate must weigh potential benefits against complications. Nonetheless, mounting evidence indicates that adrenalectomy yields improvements in blood pressure control, diabetes management, obesity metrics, and cardiovascular risk factors beyond what medical management achieves. Ultimately, the patient’s age, overall health status, and severity of metabolic derangements guide individual decision-making.
Role of Mini-Back Scope Adrenalectomy (MBSA)
Throughout recent years, Mini-Back Scope Adrenalectomy (MBSA) has emerged as the surgical technique of choice for subclinical Cushing’s syndrome. This posterior retroperitoneoscopic approach offers several advantages over traditional transperitoneal methods. Experienced surgeons can typically complete the procedure in approximately 30 minutes with patients requiring only small Band-Aids on their lower back.
MBSA enables precise function-preserving adrenalectomy while providing reduced morbidity, shorter hospital stays, and faster recovery compared to open or transabdominal approaches. Furthermore, this procedure achieves nearly 100% cure rates for cortisol-producing adrenal adenomas when performed by experienced adrenal surgeons.
The technique’s minimally invasive nature proves particularly valuable given that most subclinical Cushing’s patients are otherwise healthy individuals seeking resolution of subtle metabolic abnormalities rather than relief from dramatic symptomatology.
Monitoring protocols for mild or ambiguous cases
Conversely, conservative management remains appropriate for patients with mild biochemical abnormalities lacking clear comorbidities. For these individuals, a structured monitoring protocol provides safety while avoiding unnecessary surgery.
Retesting should occur at three-month intervals initially to verify stability of cortisol secretion. After consistent results, this interval may extend to six months. Each follow-up assessment should evaluate three key parameters: changes in tumor size, alterations in imaging characteristics, and functional status including both biochemical testing and clinical manifestations.
The conservative approach acknowledges the variable natural history of subclinical hypercortisolism—some cases remain stable for years while others progress toward overt Cushing’s syndrome. Although the ideal duration of follow-up remains undefined, monitoring must continue indefinitely given the potential for late progression.
For those eventually requiring surgery after a period of observation, postoperative glucocorticoid replacement therapy typically follows a similar protocol to overt Cushing’s syndrome, albeit with potentially shorter duration due to less profound suppression of the hypothalamic-pituitary-adrenal axis.
Postoperative Outcomes and Quality of Life Improvements
Surgical intervention for subclinical hypercortisolism produces measurable physiological improvements that often exceed the benefits of medical management alone. Postoperative outcomes demonstrate the far-reaching effects of resolving even subtle cortisol excess.
Improvements in blood pressure and glucose control
Adrenalectomy yields substantial improvements in hypertension management for patients with subclinical Cushing’s syndrome. Studies reveal that blood pressure control improves in approximately 68% of patients who undergo surgery versus just 13% of those managed conservatively. Most compelling evidence demonstrates that surgical patients often reduce their antihypertensive medication requirements from an average of 2.3 medications to 1.5 postoperatively.
Within the realm of metabolic function, glycemic control improves in about 28% of surgical patients compared to merely 3.3% of those receiving conservative treatment. Insulin resistance metrics remain stable in surgically treated patients yet worsen over time in those managed non-operatively. For individuals with diabetes at baseline, objective improvements in both fasting plasma glucose and HbA1c occur by week 12 post-intervention and persist through long-term follow-up.
Bone density recovery post-adrenalectomy
Bone health demonstrates remarkable resilience after surgical correction of subclinical Cushing’s syndrome. The prevalence of osteoporosis reaches approximately 64% in patients before intervention. After adrenalectomy, bone mineral density increases become apparent within three months, primarily in the lumbar spine.
Impressively, the percentage change in lumbar spine bone density averages 36.7% compared to 11.2% at the femoral neck over a 24-month period. Premenopausal women experience substantially greater bone recovery than their postmenopausal counterparts. First thing to remember is that complete normalization of bone density may require several years, yet progressive improvement typically continues throughout this period.
Reduction in cardiovascular risk factors
Beyond isolated physiological parameters, surgery for subclinical Cushing’s yields comprehensive cardiovascular benefits. Under these circumstances, laparoscopic adrenalectomy proves more beneficial than conservative management for normalizing multiple cardiovascular risk factors simultaneously.
Surgical intervention delivers:
- Reduced recurrence of major cardiovascular events
- Improved dyslipidemia profiles
- Decreased body weight and waist circumference
- Enhanced overall cardiovascular risk profile
As a matter of fact, these improvements translate into tangible quality of life enhancements, despite the reality that complete normalization of all quality-of-life parameters may remain elusive compared to healthy control populations. In essence, surgical cure provides the most consistent path to ameliorating the multisystem effects of subclinical hypercortisolism.

Conclusion 
Subclinical Cushing’s syndrome represents a diagnostic challenge that demands increased clinical vigilance, especially given its prevalence nearly 80 times greater than classic Cushing’s syndrome. Throughout this article, we have examined how this condition exists on a spectrum of cortisol dysregulation, often manifesting through subtle metabolic abnormalities rather than overt cushingoid features. Recognition of this entity requires attentiveness to patterns that individually appear benign yet collectively suggest underlying hypercortisolism.
The updated 2025 diagnostic criteria emphasize the importance of multiple testing modalities. Low-dose dexamethasone suppression tests, late-night salivary cortisol measurements, and ACTH assessment now follow refined protocols with specific cutoff values designed to capture even mild cases. These refined approaches address previous limitations in detection, though careful interpretation remains essential due to common confounding factors.
Clinicians should maintain heightened suspicion when encountering patients with unexplained central adiposity, treatment-resistant hypertension, osteopenia without clear risk factors, or poor glycemic control despite appropriate medication. Additionally, proximal muscle weakness, mood changes, and atraumatic vertebral fractures warrant consideration of subclinical hypercortisolism as a unifying diagnosis.
Treatment decisions balance surgical intervention against conservative management based on biochemical parameters, comorbidity severity, and patient factors. Mini-Back Scope Adrenalectomy has emerged as the preferred surgical approach, offering minimal invasiveness with excellent outcomes. Conversely, conservative management remains appropriate for milder cases, though structured monitoring protocols must continue indefinitely given the potential for disease progression.
Perhaps most compelling, surgical correction yields substantial improvements across multiple physiological systems. Blood pressure control typically improves in more than two-thirds of operated patients, with corresponding reductions in antihypertensive requirements. Likewise, glycemic parameters demonstrate meaningful improvements by week 12 post-intervention. Bone mineral density, particularly at the lumbar spine, shows remarkable recovery within months of adrenalectomy.
Undoubtedly, early identification and appropriate management of subclinical Cushing’s syndrome offer patients the opportunity to resolve metabolic derangements before irreversible complications develop. Therefore, clinicians must incorporate this condition into their differential diagnosis when evaluating patients with constellation of subtle findings that defy straightforward explanation. Though challenging to diagnose, subclinical Cushing’s syndrome responds well to appropriate intervention, making the diagnostic effort worthwhile for improved patient outcomes.
Key Takeaways
Subclinical Cushing’s syndrome is a hidden epidemic affecting 79 per 100,000 people—nearly 80 times more common than classic Cushing’s yet frequently missed due to subtle symptoms that mimic common conditions like diabetes and obesity.
- Watch for subtle warning signs: Treatment-resistant hypertension, unexplained central weight gain, early osteoporosis, and poorly controlled diabetes despite proper treatment often indicate subclinical cortisol excess.
- Use the 2025 diagnostic protocol: Low-dose dexamethasone suppression test with 1.8 μg/dL cutoff, late-night salivary cortisol, and ACTH measurement provide the most accurate detection pathway.
- Consider surgery for serious cases: Mini-Back Scope Adrenalectomy offers excellent outcomes when cortisol levels exceed 5 μg/dL after dexamethasone suppression, especially with resistant comorbidities.
- Expect dramatic post-surgical improvements: 68% of patients achieve better blood pressure control, bone density increases by 36.7% in the spine, and diabetes management significantly improves within 12 weeks.
- Monitor ambiguous cases indefinitely: Patients with mild biochemical abnormalities require lifelong surveillance as subclinical Cushing’s can progress to overt disease over time.
Early recognition and appropriate treatment of this “silent” condition can prevent irreversible complications and dramatically improve quality of life through resolution of multiple metabolic abnormalities that traditional medical management often fails to address effectively.
Frequently Asked Questions:
FAQs
Q1. What are the main differences between subclinical Cushing’s syndrome and classic Cushing’s syndrome? Subclinical Cushing’s syndrome shows biochemical evidence of cortisol excess without full clinical features. It lacks obvious signs like purple striae or moon face, instead presenting with subtle metabolic abnormalities. Classic Cushing’s has more pronounced symptoms and consistent lab abnormalities across multiple tests.
Q2. How common is subclinical Cushing’s syndrome compared to classic Cushing’s? Subclinical Cushing’s syndrome is approximately 80 times more common than classic Cushing’s syndrome. It affects an estimated 79 cases per 100,000 persons, while classic Cushing’s occurs in about 1 case per 100,000.
Q3. What are some hidden signs that might indicate subclinical Cushing’s syndrome? Key indicators include mild but persistent hypertension, unexplained central weight gain, early-onset osteoporosis, poorly controlled diabetes despite treatment, subtle fatigue and muscle weakness, mood changes, and vertebral fractures without trauma.
Q4. How is subclinical Cushing’s syndrome diagnosed in 2025? Diagnosis involves a low-dose dexamethasone suppression test (with a 1.8 μg/dL cutoff), late-night salivary cortisol measurement, and ACTH assessment. Multiple tests are often needed due to the subtle nature of the condition.
Q5. What improvements can patients expect after treatment for subclinical Cushing’s syndrome? After surgical treatment, many patients experience significant improvements in blood pressure control, diabetes management, and bone density. About 68% of patients achieve better blood pressure control, and bone density can increase by up to 36.7% in the spine within 24 months.
References:
[1] – https://www.ncbi.nlm.nih.gov/books/NBK542317/
[2] – https://academic.oup.com/jcem/article/89/3/1222/2844285?itm_medium=sidebar&itm_source=trendmd-widget&itm_campaign=The_Journal_of_Clinical_Endocrinology_%2526_Metabolism&itm_content=The_Journal_of_Clinical_Endocrinology_%2526_Metabolism_0
[3] – https://www.adrenal.com/cushing-syndrome/diagnosis
[4] – https://pubmed.ncbi.nlm.nih.gov/26884297/
[5] – https://academic.oup.com/jes/article/4/10/bvaa107/5876040
[6] – https://pmc.ncbi.nlm.nih.gov/articles/PMC4318888/
[7] – https://csrf.net/understanding-cushings/diagnostic-testing/
[8] – https://ajronline.org/doi/10.2214/AJR.16.17290
[9] – https://pmc.ncbi.nlm.nih.gov/articles/PMC6711639/
[10] – https://www.labcorp.com/test-menu/resources/corticotropin-releasing-hormone-stimulation
[11] – https://www.jstage.jst.go.jp/article/endocrj/65/4/65_EJ17-0456/_html/-char/en
[12] – https://pubmed.ncbi.nlm.nih.gov/31255204/
[13] – https://www.hcafloridahealthcare.com/locations/hospital-for-endocrine-surgery/specialties/endocrine-surgery/adrenal-surgery
[14] – https://www.sciencedirect.com/org/science/article/pii/S1479682125000670
[15] – https://pmc.ncbi.nlm.nih.gov/articles/PMC4525003/
[16] – https://consultqd.clevelandclinic.org/subclinical-cushings-syndrome-navigating-a-gray-area
[17] – https://www.sciencedirect.com/science/article/abs/pii/S1521690X12000188
[18] – https://pmc.ncbi.nlm.nih.gov/articles/PMC9202594/
[19] – https://pubmed.ncbi.nlm.nih.gov/32300953/
[20] – https://pmc.ncbi.nlm.nih.gov/articles/PMC11762609/
[21] – https://pubmed.ncbi.nlm.nih.gov/18210182/
[22] – https://csrf.net/doctors-articles/recovery/cushings-and-osteoporosis/
[23] – https://www.thelancet.com/journals/landia/article/PIIS2213-8587(14)70080-4/fulltext
[24] – https://www.nature.com/articles/hr201190
[25] – https://www.adrenal.com/blog/subclinical-cushings-syndrome-treatment-and-surgery
[26] – https://academic.oup.com/jcem/article/104/11/5325/5528225

