BTK Inhibitors in CLL: New Clinical Evidence Reshapes First-Line Treatment
Please like and subscribe if you enjoyed this video 🙂
Introduction
The treatment landscape for chronic lymphocytic leukemia (CLL), the most common leukemia in Western countries, has undergone a major shift with the advent of Bruton’s tyrosine kinase (BTK) inhibitors. These targeted therapies have replaced traditional chemoimmunotherapy as the preferred first-line approach, leading to remarkably improved patient outcomes.
Ibrutinib, the first-in-class BTK inhibitor, has been a cornerstone of this transformation. In frontline settings, it has demonstrated a seven-year progression-free survival (PFS) rate of 83 percent, underscoring its long-term efficacy. More recently, combination strategies that pair BTK inhibitors with agents like venetoclax have shown even greater promise. Notably, the fixed-duration ibrutinib-venetoclax regimen received EMA approval in August 2022 for previously untreated CLL, based on strong evidence from the GLOW and CAPTIVATE trials.
The GLOW trial, in particular, highlighted the superiority of this combination over chlorambucil-obinutuzumab, with a PFS hazard ratio of 0.216 (p<0.001) in elderly or unfit patients. Similarly, combinations involving acalabrutinib, a more selective second-generation BTK inhibitor, and venetoclax have achieved objective response rates over 92 percent, compared to 75.2 percent with FCR or BR (p<0.0001), further validating the shift toward chemotherapy-free regimens.
Beyond efficacy, newer BTK inhibitors are being developed to address limitations of earlier agents. Second-generation BTKIs offer improved selectivity and fewer cardiovascular side effects, while emerging noncovalent BTK inhibitors are designed to overcome resistance mutations that reduce the durability of first-generation therapies.
In summary, BTK inhibition has fundamentally reshaped the CLL treatment paradigm. With ongoing advances in combination strategies and next-generation agents, the future of CLL care is increasingly defined by precision, durability, and reduced toxicity.
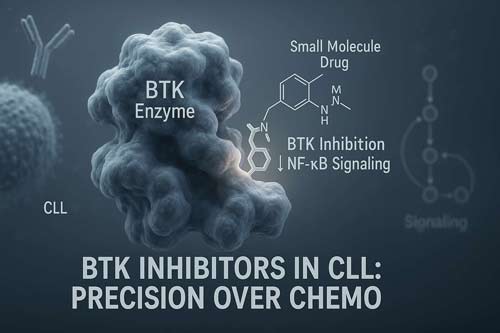
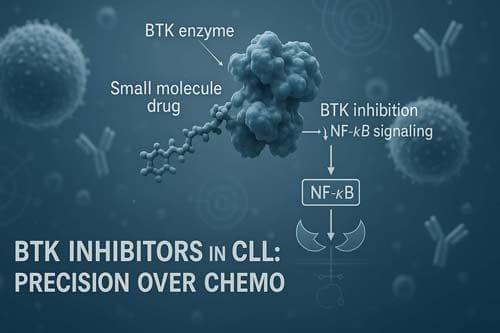
Mechanistic Rationale for BTK Inhibition in CLL
Bruton’s tyrosine kinase (BTK) stands as a critical molecular target in chronic lymphocytic leukemia (CLL) therapy. Unlike other malignancies where mutations in signaling proteins drive disease progression, BTK in CLL remains structurally normal yet functionally hyperactive. This distinction underpins the impressive efficacy of BTK inhibitor drugs in this disease.
BTK Role in B-cell Receptor Signaling
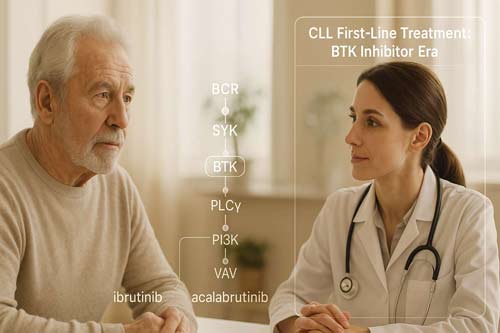
The B-cell receptor (BCR) signaling pathway represents a fundamental survival mechanism for both normal and malignant B-cells. In CLL, this pathway operates with heightened activity, creating an ideal therapeutic vulnerability. BTK functions as a pivotal intermediary in this cascade, translating external stimuli into intracellular survival and proliferation signals.
When antigen binds to surface immunoglobulin, it triggers BCR oligomerization and activation of proximal signaling molecules. Initially, Lyn kinase, constitutively associated with the CD79A/B subunits, phosphorylates immunoreceptor tyrosine-based activation motifs (ITAMs) within these structures. These phosphorylated ITAMs subsequently serve as docking sites for spleen tyrosine kinase (Syk), which further propagates the signal.
BTK occupies a strategic position downstream of Syk in this pathway. Upon Syk activation, BTK undergoes conformational changes and membrane translocation facilitated by its PH domain. At the membrane, BTK becomes accessible to substrate molecules and undergoes autophosphorylation at tyrosine 551 within its kinase domain. This activation step proves essential for signal transmission.
Once activated, BTK phosphorylates multiple downstream substrates, mainly phospholipase Cγ2 (PLCγ2). Activated PLCγ2 cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). Consequently, IP3 triggers calcium release from intracellular stores, while DAG activates protein kinase C, ultimately leading to:
- Nuclear factor kappa B (NF-κB) pathway activation
- Proliferation signal generation
- Enhanced cell survival mechanisms
- Cytokine production and release
Uniquely in CLL, BTK activation occurs through both tonic (antigen-independent) and chronic antigen-dependent stimulation. Evidence supporting this includes the presence of phosphorylated Lyn, Syk, ERK, and NF-κB subunits in unmanipulated primary CLL cells. Moreover, changes in IgM glycosylation after antigen engagement extend surface membrane display, potentially enabling interactions with tissue microenvironment components.
Impact of BTK Inhibition on CLL Cell Survival
BTK inhibition in CLL yields multi-faceted effects beyond simple kinase inactivation. BTKi CLL therapy primarily disrupts the survival signals that leukemic cells receive within their protective microenvironments. Notably, BTK inhibitors don’t cause immediate massive cell death like venetoclax or chemotherapy, but rather deprive CLL cells of essential proliferation and survival signals.
The first-in-class BTK inhibitor ibrutinib inactivates BTK by binding covalently to cysteine 481 in the ATP-binding site, irreversibly blocking phosphorylation of downstream kinases. This mechanism effectively halts BCR signal transduction, preventing activation of pathways needed for CLL cell survival, particularly NF-κB and BAFF-receptor signaling.
Beyond direct signaling disruption, BTK inhibitors profoundly affect CLL cell migration and tissue retention. All CLL/SLL patients treated with fostamatinib disodium (a SYK inhibitor affecting the same pathway) demonstrated increased lymphocytosis during the first course of treatment. Similarly, BTK inhibition thwarts leukemia cell migration toward tissue homing chemokines CXCL12 and CXCL13.
This migration inhibition explains the characteristic lymphocytosis observed during initial BTK inhibitor therapy—CLL cells previously sequestered in protective lymphoid microenvironments mobilize into the peripheral blood where they lose survival signals. As noted by Byrd et al., approximately 15-20% of patients treated with single-agent ibrutinib exhibited partial response with lymphocytosis. This phenomenon typically resolves within months as deprived cells gradually undergo apoptosis.
Clinical evidence demonstrates that BTK inhibition effectiveness correlates with the type of BCR signaling predominant in B-cell malignancies. Diseases with chronically activated BCR signaling (like CLL) show higher sensitivity to BTK inhibitors than those with tonic BCR signaling. Within CLL specifically, higher sensitivity corresponds to cases with unmutated IGHV genes, where BCR signaling plays a more prominent role in disease pathogenesis.
The selectivity profile of different BTK inhibitors determines both efficacy and side effect profiles. While ibrutinib shows distinct selectivity for BTK, it also inhibits other kinases including EGFR (IC50 5.6nM), ErbB2 (IC50 9.4nM), ITK (IC50 10.7nM), and TEC (IC50 78nM). Second-generation BTK inhibitors like acalabrutinib and zanubrutinib demonstrate improved selectivity—acalabrutinib exhibits less potent TEC inhibition (IC50 93nM vs 7nM for ibrutinib) and negligible EGFR or ITK inhibition.
This enhanced selectivity translates to improved safety profiles while maintaining efficacy. For instance, the rate of atrial fibrillation with zanubrutinib (0.1 per 100 person-months) is substantially lower than with ibrutinib (1 per 100 person-months). Therefore, the therapeutic window for newer BTK inhibitor CLL treatments continues to expand as molecular targeting becomes increasingly precise.
Synergistic Action of BTK and BCL2 Inhibitors
The combination of BTK and BCL2 inhibition represents a cornerstone advancement in targeted CLL therapy, exploiting distinct yet complementary mechanisms of action. This dual-target approach has emerged as a leading strategy among CLL latest treatments, offering deeper responses than either agent alone. Understanding the molecular interplay between these pathways illuminates why BTK inhibitor drugs paired with venetoclax yield such promising clinical outcomes.
Venetoclax-Induced Apoptosis via BCL2 Blockade
BCL2, an anti-apoptotic protein universally overexpressed in CLL cells, renders them resistant to programmed cell death by disrupting the normal balance between pro-apoptotic and anti-apoptotic molecules. As a BH3-mimetic drug, venetoclax directly antagonizes BCL2 by binding to the same pocket normally occupied by natural BH3-only proteins, thereby releasing pro-apoptotic mediators and triggering the mitochondrial apoptotic cascade.
Unlike BTK inhibitors, venetoclax induces rapid and potent apoptosis in CLL cells. Cell death becomes evident within 4 hours of exposure in vitro at concentrations achievable in vivo. In clinical settings, apoptotic CLL cells can be detected as early as 6-24 hours following a single 20-50 mg dose in some patients. This rapid cytotoxicity occurs through direct activation of the intrinsic apoptotic pathway, bypassing upstream signaling dependencies.
A crucial distinction between venetoclax and earlier BCL2 antagonists lies in its selectivity profile. Venetoclax demonstrates greater affinity for BCL2 than for related proteins BCL-W or BCL-XL. This selectivity represents a critical improvement over predecessors like navitoclax (ABT-263), which caused dose-limiting thrombocytopenia due to BCL-XL inhibition on platelets. Hence, venetoclax achieves potent anti-leukemic activity without the platelet toxicity that limited earlier BCL2 inhibitors.
Importantly, venetoclax efficacy remains intact regardless of TP53 function. In CLL cells harboring deletion 17p or TP53 mutations, venetoclax maintains equivalent cytotoxicity compared to TP53 wild-type cells. Thus, venetoclax effectively circumvents the apoptotic block associated with loss of TP53 function by directly antagonizing BCL2 downstream of TP53-mediated signaling.
BTKi-Induced BCL2 Dependence in CLL Cells
Though BTK inhibitor drugs produce profound clinical responses in CLL, they rarely induce substantial direct apoptosis in vitro. Instead, BTK inhibition creates a distinct cellular state that primes CLL cells for BCL2-targeted therapies through several complementary mechanisms.
Dynamic BH3 profiling studies reveal that BTK inhibitors selectively enhance CLL cell dependence on BCL2 without significantly altering overall mitochondrial priming. Both ibrutinib and acalabrutinib consistently increase CLL cell response to the BAD BH3 peptide (20-50% positive delta priming) without affecting response to the BIM peptide. This pattern indicates a selective increase in BCL2 dependence rather than global sensitization to apoptosis.
At the molecular level, BTK inhibition increases expression of the pro-apoptotic protein BIM. This protein shift establishes an intracellular environment primed for apoptosis execution once BCL2 is neutralized. Pre-treatment with either ibrutinib or acalabrutinib, whether ex vivo or in vivo in patients, enhances CLL cell killing by venetoclax. Remarkably, this sensitizing effect appears more pronounced with venetoclax than with conventional chemotherapeutics like fludarabine.
The synergistic relationship between these agents extends beyond simple additive effects. BTK inhibitors:
- Disrupt adhesion and prevent homing of CLL cells in lymph nodes, mobilizing them from protective microenvironments
- Downregulate MCL1 and BCLXL levels, removing alternative survival mechanisms
- Decrease CXCL12-CXCR4 signaling, enhancing CLL cell efflux into the bloodstream where they become more susceptible to venetoclax
- Reduce CD40-mediated NF-κB activation, a primary mechanism of venetoclax resistance within lymph nodes
This coordination allows the combination to target distinct CLL cell subpopulations – BTK inhibitors primarily affect dividing cells in lymphoid tissues, while venetoclax effectively eliminates resting cells in peripheral blood and bone marrow. Consequently, the combination achieves deeper remissions by addressing both proliferative and quiescent disease compartments.
Clinical implementation of this synergistic approach has yielded impressive results. In the AMPLIFY trial, the combination of acalabrutinib and venetoclax achieved overall response rates exceeding 92% compared to 75% with standard chemoimmunotherapy. Furthermore, addition of obinutuzumab to this combination raised undetectable measurable residual disease rates to approximately 95%, supporting the scientific rationale for this therapeutic approach.
Clinical Outcomes from the GLOW and CAPTIVATE Trials
Recent phase 3 trials have solidified the position of fixed-duration BTK inhibitor plus BCL2 inhibitor combinations as frontline options for chronic lymphocytic leukemia (CLL). These landmark studies provide compelling evidence that such regimens deliver superior outcomes compared to traditional approaches, especially in specific patient populations.
GLOW Trial: Ibrutinib + Venetoclax in Elderly Patients
The phase 3 GLOW trial (NCT03462719) evaluated fixed-duration ibrutinib plus venetoclax (I+V) versus obinutuzumab plus chlorambucil (Clb+O) in previously untreated elderly or comorbid CLL patients. At the 67-month follow-up analysis, I+V demonstrated a 72.7% reduction in the risk of disease progression or death compared to Clb+O (HR, 0.273; 95% CI, 0.186-0.401; P < .0001). The 66-month progression-free survival (PFS) rates were 51.7% for I+V versus 18.1% for Clb+O.
Long-term follow-up data confirmed this benefit persisted across predefined subgroups:
- At 60 months, PFS rates were 59.9% and 17.8% for I+V and Clb+O, respectively
- In patients with unmutated IGHV (uIGHV), 60-month PFS was 52.2% with I+V
- In mutated IGHV (mIGHV) patients, 60-month PFS reached 82.5% with I+V
Additionally, overall survival also showed substantial improvement with I+V, reducing the relative risk of death by 54% (HR 0.46; 95% CI, 0.27-0.79; p = 0.004). The 60-month OS rates were 81.6% with I+V versus 60.8% with Clb+O.
Beyond survival benefits, I+V demonstrated superior minimal residual disease (MRD) clearance. The best undetectable MRD rate in bone marrow by next-generation sequencing was 55.7% with I+V versus 21.0% with Clb+O (P<0.001). Furthermore, the proportion of patients with sustained uMRD in peripheral blood from 3 to 12 months after end of treatment was 84.5% for I+V compared to 29.3% for Clb+O.
CAPTIVATE Trial: MRD-Guided Fixed-Duration Therapy
CAPTIVATE (NCT02910583), a phase 2 study, employed an innovative MRD-guided approach to treatment duration. After three cycles of ibrutinib lead-in followed by 12 cycles of I+V, patients with confirmed undetectable MRD (uMRD) were randomized to placebo or continued ibrutinib.
Within the confirmed uMRD cohort, the one-year disease-free survival (DFS) rate was 95% with placebo versus 100% with ibrutinib, with no statistically meaningful difference (arm difference: 4.7% [95% CI, -1.6 to 10.9]; P = .15). This finding suggests that fixed-duration therapy may be sufficient for patients achieving deep remissions.
With longer follow-up of 61.2 months, 67% (95% CI: 59-74) of the 159 patients treated with fixed-duration I+V remained progression-free and alive at 5 years. Patients achieving uMRD in bone marrow at 3 months post-treatment demonstrated superior outcomes, with 5-year PFS rates of 84% (95% CI: 73-90) compared to 50% (95% CI: 36-62) for those not achieving uMRD.
Toxicity-Free PFS as a New Endpoint
A novel endpoint, toxicity-free PFS—defined as freedom from disease progression/death and grade 3 or higher treatment-emergent adverse effects (TEAEs)—offers a more comprehensive assessment of treatment benefit. In GLOW, median toxicity-free PFS was 51.6 months with I+V versus 30.2 months with Clb+O.
In a 64-month follow-up analysis, researchers found that although patients receiving 15 months of I+V spent slightly more time in the grade 3/4 toxicity state versus those receiving 6 months of Clb+O (2 vs 1 month), the I+V arm spent substantially more time in TEAE-free PFS (50 vs 30 months). This translates to a 20-month improvement in quality survival time—a clinically meaningful benefit.
Regarding safety, adverse events grade 3 or greater occurred in 75.5% of I+V patients versus 69.5% with Clb+O, with neutropenia being most common in both arms (34.9% and 49.5%, respectively). Despite the higher rate of adverse events, the toxicity-free PFS analysis indicates that the benefits of I+V outweigh the risks when compared to standard chemoimmunotherapy.
AMPLIFY Trial: Acalabrutinib + Venetoclax ± Obinutuzumab
The AMPLIFY trial expands the frontline BTK inhibitor CLL armamentarium by evaluating fixed-duration acalabrutinib-venetoclax regimens against standard chemoimmunotherapy. As the first randomized study assessing a second-generation BTKi with venetoclax, AMPLIFY provides valuable insights into the comparative efficacy of these newer targeted combinations.
Trial Design and Patient Stratification
AMPLIFY (ACE-CL-311; NCT03836261) enrolled adults with treatment-naïve CLL without del(17p) or TP53 mutations. Patients required Eastern Cooperative Oncology Group performance status ≤2 to participate. In this open-label phase 3 trial, 867 patients were randomized 1:1:1 to receive:
- Acalabrutinib-Venetoclax (AV): Oral acalabrutinib 100 mg twice daily (cycles 1-14); oral venetoclax daily (cycles 3-14 with 5-week dose ramp-up)
- Acalabrutinib-Venetoclax-Obinutuzumab (AVO): AV plus intravenous obinutuzumab 1000 mg on cycles 2 (days 1, 8, 15) and 3-7 (day 1)
- Chemoimmunotherapy: Investigator’s choice of FCR or BR per standard dosing for 6 cycles
The study population had a median age of 61 years, with 64.5% male participants and 58.6% harboring unmutated IGHV. Approximately 63% of patients came from Europe, and 15% exhibited complex karyotype. Within the chemoimmunotherapy arm, patients received either FCR (n=143) or BR (n=147).
PFS and ORR Metrics Compared to FCR/BR
At a median follow-up of 41 months, both acalabrutinib-containing regimens demonstrated superior progression-free survival compared to chemoimmunotherapy. The hazard ratios versus FCR/BR were 0.65 (p=0.0038) for AV and 0.42 (p<0.0001) for AVO. Neither acalabrutinib arm reached median PFS, whereas the FCR/BR arm showed median PFS of 47.6 months.
The 36-month PFS rates underscored this advantage, with rates of 76.5% for AV, 83.1% for AVO, and 66.5% for FCR/BR. In parallel, objective response rates were markedly higher with both acalabrutinib combinations (92.8% for AV, 92.7% for AVO) versus chemoimmunotherapy (75.2%, p<0.0001 for both comparisons).
Concerning overall survival, the AV arm showed a positive trend over FCR/BR with a hazard ratio of 0.33 (p<0.0001). When censoring for COVID-19 deaths, both experimental arms maintained their survival advantage with hazard ratios of 0.27 for AV and 0.47 for AVO versus chemoimmunotherapy.
Minimal residual disease clearance rates were highest in the AVO arm, with 95% of evaluable patients achieving undetectable MRD versus 45% with AV and 72.9% with FCR/BR.
Subgroup Analysis: IGHV Mutation Status
IGHV mutation status proved a major factor in treatment outcomes across arms. Results from the AMPLIFY trial suggest that adding obinutuzumab to acalabrutinib-venetoclax offered particular benefit for patients with unmutated IGHV.
In comparison, data from a phase II study of acalabrutinib-venetoclax-obinutuzumab with MRD-guided therapy duration showed four-year PFS rates of 93% in patients with mutated IGHV versus 81% in those with unmutated IGHV. This highlights how targeted combinations may help narrow the historically poor prognosis associated with unmutated IGHV.
The favorable toxicity profile of acalabrutinib combinations merits attention alongside efficacy data. The most common serious adverse event across all arms was neutropenia, while rates of BTKi-associated complications including tumor lysis syndrome, atrial fibrillation, and serious hypertension remained low in the acalabrutinib arms.
Through these comprehensive endpoints, AMPLIFY establishes fixed-duration acalabrutinib-venetoclax combinations as compelling alternatives to chemoimmunotherapy for frontline CLL treatment, offering a new opportunity to optimize BTKi CLL therapy based on individual patient characteristics.
Emerging BTKi Combinations in Early-Phase Trials
Beyond established BTK inhibitor CLL regimens, several early-phase trials are exploring novel combinations that aim to overcome resistance mechanisms and achieve deeper remissions. These emerging protocols utilize next-generation BTK inhibitors alongside potent BCL2-targeting agents to maximize therapeutic potential.
Pirtobrutinib + Venetoclax + Obinutuzumab (NCT05536349)
The phase 2 trial of pirtobrutinib (a non-covalent BTK inhibitor) combined with venetoclax and obinutuzumab demonstrates impressive preliminary efficacy in previously untreated CLL. At a median follow-up of 11.9 months, this triplet regimen induced exceptionally deep responses. The study protocol administered pirtobrutinib 200mg daily starting cycle 1 day 1 continuously until cycle 13, with standard obinutuzumab for 6 cycles and venetoclax (following standard ramp-up) from cycle 2 through cycle 13.
Response assessment revealed extraordinary minimal residual disease (MRD) clearance rates. Indeed, among patients completing 7 cycles of treatment (n=66), peripheral blood undetectable MRD at 10⁻⁶ sensitivity (uMRD6) reached 79%, with 93% achieving uMRD4. Even more impressive, patients completing 13 cycles (n=41) demonstrated 100% uMRD4 rates and 85% uMRD6 rates in peripheral blood.
Bone marrow assessments showed corresponding depth of response. After 7 cycles, 64% of patients achieved bone marrow uMRD6 and 91% reached uMRD4. Upon completion of 13 cycles, these rates improved to 81% uMRD6 and 96% uMRD4 in bone marrow. Of note, this triplet appears effective across risk groups, with 77% of enrolled patients having unmutated IGHV and 12% harboring del(17p)/TP53 mutations.
Safety data revealed predominantly hematologic toxicities, with grade 3-4 neutropenia occurring in 58% and thrombocytopenia in 18% of patients. In all, 21% required pirtobrutinib dose reduction and 31% needed venetoclax dose adjustments, primarily due to neutropenia. As of latest follow-up, no patient has progressed or died.
Zanubrutinib + Sonrotoclax in CELESTIAL-TNCLL Trial
The CELESTIAL-TNCLL study (NCT06073821) represents another promising frontier in BTK inhibitor CLL therapy, investigating zanubrutinib in combination with sonrotoclax, a next-generation BCL2 inhibitor. Unlike other combinations, this approach pairs two next-generation targeted agents.
Sonrotoclax (BGB-11417) exhibits greater selectivity and higher potency against BCL2 than venetoclax, coupled with a shorter half-life that may allow more flexible dosing. In parallel, zanubrutinib demonstrated superior progression-free survival with fewer cardiac adverse events compared to ibrutinib in previous head-to-head trials.
This randomized, open-label phase 3 study will enroll approximately 640 patients with treatment-naïve CLL requiring therapy per iwCLL criteria. The experimental arm receives 3 cycles of zanubrutinib monotherapy (320 mg daily), followed by zanubrutinib plus sonrotoclax for 12 cycles. The control arm receives standard venetoclax plus obinutuzumab for 12 cycles.
Preliminary phase 1 data from the sonrotoclax-zanubrutinib combination show extraordinary early efficacy. In treatment-naïve CLL patients, the combination achieved 100% overall response rates with complete response rates of 41-42% across dose cohorts. Even more impressive, week 48 best blood uMRD4 rates reached 79% with 160mg sonrotoclax and 90% with 320mg sonrotoclax.
From a safety perspective, no laboratory or clinical tumor lysis syndrome events occurred in early studies, and only one patient discontinued the combination due to adverse events. The most common side effects included neutropenia (41%), contusion (38%), and diarrhea (29%, predominantly grade 1).
Both these emerging BTKi combinations represent promising advances that may ultimately expand treatment options for patients with CLL. Above all, the impressive MRD clearance rates suggest potential for deeper, more durable remissions than currently approved regimens.
Resistance Mutations and Non-Covalent BTK Inhibitors
Despite the clinical success of BTK inhibitor drugs in CLL management, resistance inevitably emerges as treatment continues. Understanding these resistance mechanisms has spurred development of novel agents capable of overcoming these challenges.
C481S and T474I Mutation Profiles
Long-term BTK inhibitor therapy selectively pressures malignant B-cells, resulting in genetic adaptations that limit drug efficacy. The most frequent resistance mechanism involves mutations at the cysteine 481 residue, predominantly C481S, which occurs in over 90% of ibrutinib-resistant cases. This mutation fundamentally alters how covalent BTK inhibitors (cBTKis) interact with their target—reducing binding avidity and rendering the previously irreversible interaction reversible.
Interestingly, resistance patterns vary across different cBTKis. While C481S remains predominant with all agents, secondary mutations emerge with distinct frequencies. In acalabrutinib-treated patients, T474I gatekeeper mutations appear in 29% of resistant cases, often alongside C481S mutations. Likewise, zanubrutinib resistance frequently involves L528W kinase-impaired mutations, which were detected in all four patients who progressed in one small study.
Additonally, kinase-dead BTK mutants—which cannot autophosphorylate Y223 yet maintain downstream signaling—are uncommon with ibrutinib but relatively frequent with zanubrutinib or pirtobrutinib therapy. This distinction possibly stems from ibrutinib’s off-target effects on HCK, potentially explaining how kinase-dead BTK mutants continue signal transduction.
Pirtobrutinib Activity in BTK-Mutant CLL
Pirtobrutinib represents a breakthrough as the first non-covalent BTK inhibitor that binds distant from the C481 residue. This structural innovation enables activity regardless of C481 mutation status. Data from the BRUIN trial demonstrated an impressive 82% overall response rate among 247 cBTKi-treated patients.
Also, pirtobrutinib maintains effectiveness across resistance profiles, achieving 73.9% response rates in patients without BTK C481 mutations and 55.6% responses even in those with PLCG2 mutations. The drug shows particular potency against specific mutation types, with 96% response rates in patients harboring T474x mutations and 79% in those with L528W mutations.
BTK Degraders: NX-2127 and BGB-16673
BTK degraders represent the newest frontier in overcoming resistance. Unlike inhibitors that merely block enzyme function, degraders eliminate the BTK protein entirely through the ubiquitin-proteasome pathway. This approach potentially addresses all mutation-based resistance mechanisms.
Early clinical trials of BGB-16673 show promising efficacy in heavily pretreated patients, with a 93.8% overall response rate at the 200mg dosage, including complete responses. Particularly noteworthy, this agent demonstrates activity in Richter Transformation—an aggressive CLL variant—with 58.3% of patients responding.
Other BTK degraders under investigation include NX-2127 and NX-5498, both showing encouraging preliminary results in patients who have exhausted multiple prior therapies. These agents represent a potential solution for patients with limited remaining options after developing resistance to both covalent and non-covalent BTK inhibitors.
Guideline Updates and Regulatory Shifts
The regulatory landscape for BTK inhibitor CLL treatment continues to evolve as emerging clinical evidence reshapes practice recommendations. Recent updates reflect shifting priorities toward fixed-duration combinations and improved safety profiles.
NCCN Category 1 and 2A Recommendations
National Comprehensive Cancer Network (NCCN) guidelines have undergone substantial revisions based on accumulating evidence from pivotal trials. Zanubrutinib has moved from category 2A to category 1 preferred status for first-line treatment in patients without del(17p)/TP53 mutations. Similarly, venetoclax plus obinutuzumab was elevated to category 1 for patients younger than 65 years without remarkable comorbidities.
In contrast, ibrutinib was repositioned from preferred regimens to “other recommended regimens” with a specific footnote regarding its toxicity profile. Patients receiving ibrutinib now require baseline cardiac function assessment prior to initiation.
Furthermore, the NCCN has added acalabrutinib plus venetoclax (with or without obinutuzumab) as a category 1 preferred regimen for patients without del(17p)/TP53 mutations and category 2A for those with these mutations. Concurrently, ibrutinib plus venetoclax was upgraded from category 2B to category 2A status, reflecting growing confidence in this combination.
For relapsed/refractory disease, pirtobrutinib received category 2A recommendations as second-line or third-line therapy after BTKi failure, based on data from the BRUIN study.
EMA vs FDA Approval Status of BTKi + BCL2i Regimens
Interestingly, European and American regulatory approvals for BTKi-BCL2i combinations reveal distinct approaches. The European Commission granted approval to ibrutinib plus venetoclax as a fixed-duration regimen for previously untreated CLL in August 2022, based on the GLOW and CAPTIVATE trials. More recently, acalabrutinib plus venetoclax (with or without obinutuzumab) received European approval for treatment-naïve CLL.
Meanwhile, in the United States, these combinations remain unapproved by the FDA despite their inclusion in NCCN guidelines. This regulatory divergence creates a situation where American practitioners must rely on guideline recommendations rather than formal FDA approvals when considering these regimens.
Both European approvals emphasize the fixed-duration approach, which according to clinical investigators, “helps with adherence during the treatment period” and “provides patients and physicians more flexibility in managing this incurable blood cancer”.
Clinical Implications for First-Line CLL Management
Current first-line management of chronic lymphocytic leukemia presents clinicians with multiple effective options, necessitating careful assessment of individual patient characteristics to optimize outcomes. The decision-making process now extends beyond simple efficacy metrics to encompass quality of life considerations, genomic risk factors, and treatment duration preferences.
Time-Limited vs Continuous Therapy Considerations
The emergence of fixed-duration regimens has created a fundamental paradigm shift in CLL treatment. Fixed-duration venetoclax-based therapies offer treatment-free intervals, whereas BTK inhibitors traditionally require continuous administration until disease progression or intolerable toxicity. Concerning venetoclax-obinutuzumab, this one-year regimen produces durable remissions while allowing patients extended treatment-free periods. In comparison, BTK inhibitors acalabrutinib and zanubrutinib typically follow a continuous treatment schedule.
Each approach carries distinct advantages. Continuous BTK inhibitor therapy proves logistically simpler, avoiding the tumor lysis monitoring required with venetoclax initiation. In contrast, fixed-duration therapy offers:
- Freedom from chronic medication management
- Potentially lower cumulative toxicity burden
- Possibly reduced drug resistance development
Despite initial intuitions, extended follow-up data from the GLOW trial revealed that fixed-duration ibrutinib plus venetoclax provided a toxicity-free progression-free survival of 51.6 months versus 30.2 months with chlorambucil-obinutuzumab. Equally important, multiple trials of BTK inhibitor plus venetoclax combinations demonstrate that one year of treatment can achieve deep molecular remissions with stable MRD levels on serial analysis.
Patient Selection Based on Genomic Risk
Genomic risk stratification remains crucial for optimal therapy selection. Of course, patients with TP53 aberrations (deletion 17p or TP53 mutations) respond poorly to conventional chemoimmunotherapy, with median PFS approximately one year with FCR compared to six-year PFS of ~60% with ibrutinib. For this reason, continuous BTK inhibitor therapy has traditionally been recommended for TP53-aberrant cases.
Nevertheless, emerging evidence suggests that fixed-duration BTK inhibitor plus venetoclax combinations may provide effective options even for genomic high-risk patients. In the CLL14 study, venetoclax-obinutuzumab produced longer PFS in patients with del(17p) and/or TP53 mutation compared to chemoimmunotherapy (5-year PFS 40.6% vs 15.6%).
IGHV mutation status further refines treatment selection. A somewhat counterintuitive finding from ibrutinib-venetoclax studies shows patients with unmutated IGHV achieve higher rates of undetectable MRD than those with mutated IGHV. With this intention, treatment selection increasingly considers how genomic features predict response depth and durability rather than simply excluding certain therapies.

Conclusion
BTK inhibitor (BTKi) therapy has revolutionized the treatment landscape of chronic lymphocytic leukemia (CLL), moving away from traditional chemoimmunotherapy toward targeted regimens with superior efficacy and more manageable toxicity. Both continuous single-agent BTKi and fixed-duration BTKi-venetoclax combinations have demonstrated significant improvements in progression-free survival (PFS), driven by their complementary mechanisms, BTK inhibition disrupts B-cell receptor signaling, while venetoclax induces apoptosis in primed CLL cells via BCL2 inhibition.
Key trials, including GLOW, CAPTIVATE, and AMPLIFY, have firmly established BTKi-venetoclax combinations as frontline options across a broad range of patients. For example, GLOW reported a 72.7% risk reduction in progression or death with ibrutinib-venetoclax versus chlorambucil-obinutuzumab. Similarly, AMPLIFY demonstrated a 92.7% overall response rate with acalabrutinib-venetoclax compared to 75.2% with chemoimmunotherapy. These regimens also achieved deeper remissions and higher rates of undetectable minimal residual disease (uMRD), supporting the possibility of longer treatment-free intervals.
However, resistance remains a clinical challenge. Mutations such as C481S and T474I disrupt BTKi binding, necessitating next-generation approaches. Non-covalent BTK inhibitors like pirtobrutinib retain activity against resistant clones, while BTK degraders offer an alternative by targeting the BTK protein for elimination, potentially overcoming mutation-driven resistance altogether.
Regulatory frameworks are adapting to this rapidly evolving evidence base. While European approvals for BTKi-venetoclax combinations are in place, FDA approvals trail behind NCCN guideline recommendations, which now favor second-generation BTK inhibitors and fixed-duration combinations for many patient subsets.
Treatment selection should consider disease biology (e.g., TP53 abnormalities, IGHV mutation status), comorbidities, and patient preference. Fixed-duration regimens may offer lower cumulative toxicity and defined treatment endpoints, whereas continuous BTKi therapy avoids the complexities of venetoclax ramp-up and monitoring. Notably, emerging data suggest that even high-risk patients, historically managed with indefinite BTKi therapy, may benefit from time-limited combinations.
Looking ahead, early-phase studies (e.g., pirtobrutinib-venetoclax-obinutuzumab and zanubrutinib-sonrotoclax) are showing exceptional MRD clearance, pointing toward increasingly precise, chemotherapy-free strategies. As these targeted combinations mature, they are redefining the goals of therapy, not only prolonging survival but also potentially achieving functional cures.
In summary, BTKi-based therapy stands at the forefront of precision medicine in CLL, offering durable disease control, expanded treatment options, and a clear path toward more personalized, patient-centered care.
Frequently Asked Questions:
FAQs
Q1. What are the latest advancements in first-line treatment for CLL? Recent clinical trials have shown that combinations of BTK inhibitors (like ibrutinib or acalabrutinib) with venetoclax are highly effective as first-line treatments for CLL. These targeted therapy combinations often achieve deeper and more durable remissions compared to traditional chemoimmunotherapy.
Q2. How do BTK inhibitors and BCL2 inhibitors work together in CLL treatment? BTK inhibitors disrupt B-cell receptor signaling pathways critical for CLL cell survival, while simultaneously priming cells for BCL2-dependent apoptosis. BCL2 inhibitors like venetoclax then trigger this programmed cell death, leading to synergistic anti-leukemic effects.
Q3. What are the advantages of fixed-duration therapy in CLL? Fixed-duration regimens, such as one-year courses of BTK inhibitor plus venetoclax, offer several benefits including treatment-free intervals, potentially lower cumulative toxicity, and possibly reduced development of drug resistance while still achieving deep molecular remissions.
Q4. How are emerging resistance mechanisms being addressed in CLL therapy? Novel agents like pirtobrutinib, a non-covalent BTK inhibitor, are being developed to overcome common resistance mutations like C481S. Additionally, BTK degraders represent a promising new class of drugs that may circumvent mutation-based resistance by eliminating the BTK protein entirely.
Q5. What factors should be considered when selecting CLL treatment? Treatment selection should balance multiple factors including genomic risk profiles (e.g., TP53 aberrations, IGHV mutation status), patient comorbidities, and preferences regarding treatment duration. The choice between continuous therapy and fixed-duration approaches may depend on these individual patient characteristics.
References:
[1] – https://ashpublications.org/blood/article/144/Supplement 1/1011/530874/Combined-Pirtobrutinib-Venetoclax-and-Obinutuzumab
[2] – https://ashpublications.org/blood/article/141/26/3137/495695/Pirtobrutinib-a-new-hope-for-patients-with-BTK
[3] – https://ashpublications.org/blood/article/144/Supplement 1/1009/530876/Fixed-Duration-Acalabrutinib-Plus-Venetoclax-with
[4] – https://ascopubs.org/doi/10.1200/JCO.2024.42.16_suppl.TPS7087
[5] – https://ashpublications.org/blood/article/144/Supplement 1/3257.1/530221/CELESTIAL-TNCLL-An-Ongoing-Open-Label
[6] – https://ashpublications.org/bloodadvances/article/8/9/2300/515303/BTK-inhibitors-in-CLL-second-generation-drugs-and
[7] – https://www.ajmc.com/view/recent-nccn-update-moves-zanubrutinib-ahead-of-ibrutinib-in-cll-sll-based-on-toxicity-profile
[8] – https://mcpress.mayoclinic.org/managing-cll/chronic-lymphocytic-leukemia-cll-understanding-the-difference-between-fixed-duration-and-continuous-treatment/
[9] – https://www.appliedclinicaltrialsonline.com/view/amplify-trial-calquence-venetoclax-survival-untreated-cll
[10] – https://www.pharmacytimes.com/view/the-phase-3-amplify-trial-assesses-a-new-treatment-paradigm-in-patients-with-treatment-naive-cll
[11] – https://ascopubs.org/doi/10.1200/JCO-24-02503
[12] – https://www.onclive.com/view/combination-regimens-expand-cll-treatment-paradigm
[13] – https://www.onclive.com/view/first-line-pirtobrutinib-plus-venetoclax-obinutuzumab-generates-high-umrd-rates-in-cll
[14] – https://ashpublications.org/blood/article/144/Supplement 1/1012/531224/Sonrotoclax-and-Zanubrutinib-as-Frontline
[15] – https://ashpublications.org/blood/article/144/10/1061/516126/Mutational-profile-in-previously-treated-patients
[16] – https://www.sciencedirect.com/science/article/pii/S0006497125001375
[17] – https://www.cancernetwork.com/view/pirtobrutinib-effective-in-heavily-pretreated-btk-mutated-cll?utm_source=dataLayer&medium=cmi_oss&campaign=newsDeck
[18] – https://www.cancernetwork.com/view/btk-degrader-bgb-16673-shows-promise-in-r-r-wm-and-cll-sll
[19] – https://cllsociety.org/2025/04/early-insights-into-novel-btk-degrader-to-treat-relapsed-cll/
[20] – https://www.vjhemonc.com/latest-advances-in-time-limited-btk-and-bcl2-inhibitor-combinations-for-the-frontline-treatment-of-cll/
[21] – https://cllsociety.org/2023/07/new-nccn-guidelines-recommend-pirtobrutinib/
[22] – https://www.jnj.com/media-center/press-releases/european-commission-approves-imbruvica-ibrutinib-in-a-fixed-duration-combination-regimen-for-adult-patients-with-previously-untreated-chronic-lymphocytic-leukemia-cll
[23] – https://www.onclive.com/view/acalabrutinib-based-regimens-receive-european-approval-in-frontline-cll
[24] – https://lymphomahub.com/medical-information/combination-therapies-for-cll-btki-bcl2i
[25] – https://pmc.ncbi.nlm.nih.gov/articles/PMC10891251/
[26] – https://www.cancernetwork.com/view/firstline-btki-monotherapy-in-patients-with-cll-4-year-follow-up-data-from-elevate-tn
[27] – https://www.onclive.com/view/btk-inhibitors-vs-venetoclax-considerations-for-frontline-treatment-selection-in-cll

