Why Diagnostic Stewardship in the ED Actually Reduces Medical Errors

Introduction
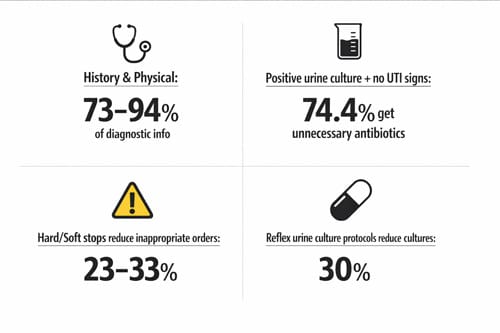
Diagnostic stewardship represents a critical yet underutilized approach to reducing medical errors in emergency medicine. In fact, history and physical examination alone provide 73%-94% of the information needed for accurate diagnosis, with diagnostic testing contributing far less than most practitioners assume. Despite this reality, we continue to see alarming rates of overtesting and misdiagnosis across emergency departments nationwide.
The consequences of poor diagnostic practices are substantial. A striking 74.4% of emergency department patients with positive urine cultures but no documented signs of UTI receive unnecessary antimicrobial therapy. Furthermore, only 33.7% of hospital resident physicians can correctly differentiate asymptomatic bacteriuria from true infection. These knowledge gaps contribute to antimicrobial therapy being prescribed in approximately 50% of asymptomatic bacteriuria cases documented in the clinical literature.
The Centers for Disease Control and Prevention (CDC) recognizes this challenge. In 2021, the CDC Division of Healthcare Quality Promotion, along with thought leaders, released a white paper emphasizing diagnostic stewardship’s importance for healthcare-associated infections, antimicrobial resistance, and sepsis management. Their guidance highlights several benefits of proper diagnostic stewardship:
- Reduction in overuse of blood cultures, which currently leads to low yields and high contamination rates • Decreased unnecessary antibiotic usage and its associated complications • Shorter hospitalization periods and reduced mortality rates • More targeted treatment approaches through syndromic testing that can provide pathogen-specific results in about an hour
In this article, we examine how diagnostic stewardship practices specifically reduce medical errors in emergency department settings. We explore common diagnostic errors, effective interventions across the testing process, real-world case studies, and practical implementation guidelines for ED practitioners.
Key Takeaways
Diagnostic stewardship in emergency departments is a systematic approach to reducing medical errors by implementing evidence-based testing protocols that improve patient outcomes while avoiding unnecessary treatments.
- History and physical examination provide 73-94% of diagnostic information needed, yet 74% of ED patients with positive urine cultures receive unnecessary antibiotics
- Reflex testing protocols reduce unnecessary cultures by 30% while hard/soft stops in electronic systems decrease inappropriate test ordering by 23-33%
- Machine learning models achieve >94% negative predictive value for identifying low-risk bacteremia cases, enabling safer withholding of blood cultures
- Successful implementation requires multidisciplinary teams, physician champions, and careful EMR design to avoid alert fatigue while maintaining clinical effectiveness
- Diagnostic stewardship reduces healthcare-associated infections by 30-50% and decreases antimicrobial misuse through more accurate diagnosis of common conditions
The evidence demonstrates that diagnostic stewardship transforms emergency medicine from reactive testing to proactive, evidence-based decision-making. This approach not only prevents medical errors but also reduces costs, shortens hospital stays, and improves antibiotic stewardship outcomes across healthcare systems.
What Is Diagnostic Stewardship and Why Does It Matter in the ED
The concept of diagnostic stewardship has emerged as a pivotal approach in modern healthcare systems. Initially developed as a laboratory activity focused on specimen collection and processing, it has evolved into a comprehensive quality improvement process involving multidisciplinary teams [1].
Diagnostic stewardship definition and CDC core elements
Diagnostic stewardship encompasses the systematic application of specific, evidence-based actions that guide clinicians toward selecting the right test for the right patient at the right time [2]. According to the CDC, this approach ensures that diagnostic tests are ordered, interpreted, communicated, and acted upon appropriately [2]. The process extends across the entire diagnostic pathway—from test ordering to result interpretation and clinical decision-making.
The CDC has established core elements for diagnostic excellence programs that hospitals can implement to improve diagnostic processes:
- Leadership commitment and accountability
- Expert consultation and oversight
- Tracking and reporting diagnostic processes
- Education and training for healthcare providers
- Implementation of targeted interventions
- Interdisciplinary collaboration between clinicians and laboratory professionals [2]
Through these elements, diagnostic stewardship can reduce certain healthcare-associated infection rates by 30-50% while simultaneously improving antibiotic use by reducing misdiagnosis of common infections [2].
How diagnostic stewardship differs from antimicrobial stewardship
Although related, diagnostic and antimicrobial stewardship serve distinct yet complementary functions. While antimicrobial stewardship focuses on optimizing antibiotic prescribing practices—ensuring antibiotics are used only when necessary and appropriately [3]—diagnostic stewardship centers on ensuring that treatment decisions about antimicrobial use are based on accurate, timely diagnostic results [4].
The relationship becomes clear when considering the following distinctions:
Antimicrobial stewardship primarily addresses how antibiotics are prescribed and used once a diagnosis has been made. Conversely, diagnostic stewardship focuses on whether antibiotics are truly needed at all, based on accurate diagnosis [4]. Consequently, diagnostic stewardship enhances the effectiveness of antimicrobial stewardship by providing the foundation for appropriate treatment decisions.
Additionally, diagnostic stewardship involves a broader range of stakeholders, often including healthcare epidemiology personnel, clinical and medical microbiologists, alongside the antibiotic stewardship team [1].
Why emergency departments are high-risk for diagnostic errors
Emergency departments present particularly challenging environments for accurate diagnosis. The National Academy of Medicine has identified diagnostic error as a “blind spot” for modern medicine, with EDs considered high-risk settings for such errors [5]. Several factors contribute to this elevated risk:
First, the ED environment features time constraints, high patient volumes, and frequent interruptions—all of which can compromise cognitive processes essential for accurate diagnosis. Meanwhile, patients often present with undifferentiated symptoms at early disease stages, making pattern recognition more difficult.
Moreover, the diagnostic process typically unfolds over time and across multiple care locations, beginning with symptom development at home and potentially progressing through ambulatory settings before reaching the ED [2]. This fragmented care journey complicates information gathering and continuity of care.
Given these challenges, diagnostic stewardship interventions hold particular value in emergency settings, where they can significantly reduce misdiagnosis rates, prevent unnecessary antimicrobial use, and ultimately improve patient outcomes through more accurate and timely diagnoses.

Common Diagnostic Errors in the ED and Their Consequences 
Emergency departments face unique diagnostic challenges that can lead to serious medical errors. A systematic review found that an estimated 5.7% of all ED visits involve at least one diagnostic error, with 0.3% resulting in serious harms and 0.2% in preventable deaths [6]. These errors often stem from cognitive failures, primarily in clinical decision-making and judgment.
Overdiagnosis of asymptomatic bacteriuria (ASB)
Overdiagnosis of ASB represents one of the most common errors in emergency medicine. In EDs, up to 52% of women with genitourinary symptoms receive incorrect UTI diagnoses [7]. This pattern of overdiagnosis occurs primarily because:
- Abnormal urinalysis results appear in 92% of patients but poorly predict true infection [7]
- Nearly one-quarter (24%) of patients diagnosed with UTIs have no documented UTI-related symptoms [7]
The consequences extend beyond unnecessary antibiotic use. Notably, 64% of patients with missed sexually transmitted infections were erroneously diagnosed with UTIs instead [7]. This misdiagnosis chain continues throughout hospitalization—81.8% of patients started on antibiotics for incorrectly diagnosed UTIs by emergency physicians remained on those antibiotics by day 3 of hospitalization [7].
False-positive Clostridioides difficile infections (CDI)
Laboratories that use only PCR nucleic acid amplification tests (NAATs) for C. difficile testing frequently misdiagnose colonization as infection. PCR tests detect the toxin gene without confirming the presence of free toxin, leading to overestimation of CDI incidence [8]. Moreover, 3-26% of hospitalized patients are asymptomatic carriers, contributing substantially to false positive results [9].
Yet, current evidence suggests standard of care (SOC) practices simultaneously underdiagnose 40.4% of true CDI cases while overdiagnosing 16% [8]. This testing paradox highlights how diagnostic error affects both ends of the spectrum—missed diagnoses and overdiagnosis—ultimately resulting in 24.4% fewer cases being identified than under optimized testing protocols [8].
Contaminated blood cultures and unnecessary treatment
Blood culture contamination represents a persistent challenge in emergency settings. One study found baseline contamination rates of 4.3%, well above the recommended 3% benchmark [10]. Contaminated cultures typically contain skin flora, such as coagulase-negative Staphylococcus, Corynebacterium species, and Bacillus species [3].
The clinical and financial impacts are substantial:
- Each contaminated culture costs healthcare systems approximately $4,538 [3]
- Patients with contaminated cultures experience hospital stays 2 days longer than those with negative cultures [3]
- Patients undergo unnecessary antibiotic treatments, additional ED visits, hospitalizations, laboratory tests, and invasive procedures [10]
Misdiagnosis of ventilator-associated pneumonia (VAP)
VAP represents a diagnostic conundrum in emergency and critical care settings. Among patients clinically diagnosed with VAP, only 40-50% actually have the condition when confirmed by microbiological studies [1]. Nevertheless, in one study, 58.4% of potential VAP cases were ultimately determined not to have VAP [11].
Despite this reality, antibiotics were continued in 76% of patients without VAP on day 3 of treatment [11]. This overtreatment generated 1,183 excess antibiotic days for patients without any indication for antibiotics [11]. The error stems partly from difficulties in diagnosis—VAP guidelines across Europe and America differ substantially in their diagnostic approaches and empiric antibiotic selection [1].
Implementing diagnostic stewardship protocols offers a pathway to reduce these common ED errors by standardizing testing criteria, improving specimen collection techniques, and establishing clearer result interpretation guidelines.
How Diagnostic Stewardship Interventions Work in Practice
Effective diagnostic stewardship operates across three distinct phases of the testing process, each offering specific opportunities to reduce errors and improve patient outcomes. This structured approach systematically addresses weaknesses in how tests are ordered, processed, and reported.
Ordering phase: clinical decision support and test restrictions
The ordering phase represents a critical intervention point where computerized clinical decision support (CCDS) tools modify provider behavior. These tools include:
- Electronic “soft stops” that allow providers to proceed after viewing alerts about appropriate criteria
- Electronic “hard stops” that prevent order placement unless specific criteria are met
- Best practice alerts (BPAs) guide at the point of order entry
- Required indication documentation for test ordering [5]
Studies demonstrate the impact of these interventions. Hard stops for C. difficile testing resulted in an adjusted 33% reduction in test ordering, while soft stops achieved a 23% reduction [4]. Another approach eliminated nurse-driven automatic blood culture orders for sepsis alerts, requiring treating providers to place orders based on clinical judgment rather than automated protocols [12]. This intervention reduced blood culture utilization from 224.3 to 99.7 cultures per 1,000 patient-days while improving positivity rates from 7.07% to 8.65% [12].
Processing phase: reflex testing and lab criteria enforcement
During the analytical phase, laboratories can implement protocols that determine which specimens receive full processing. Reflex testing—where tests are performed only after pre-specified criteria are met—represents a cornerstone strategy [13]. For urine cultures, this approach limits processing to specimens with evidence of inflammation on urinalysis.
One study implemented reflex urine culture testing in adult ICUs based on pyuria (WBC count >10/hpf), resulting in a 30% reduction in processed cultures [4]. This approach has a negative predictive value exceeding 90% for ruling out infection in the absence of pyuria [14]. For C. difficile testing, two-step algorithms combining methods can significantly reduce false positives from asymptomatic colonization [4].
Reporting phase: selective reporting and EMR nudges
The post-analytical phase focuses on interpreting and communicating results to guide appropriate clinical decisions. Effective strategies include:
Selective or cascade reporting of antimicrobial susceptibilities suppresses secondary options unless primary narrow-spectrum agents show resistance [14]. This approach has successfully altered prescribing practices for suspected UTIs [4].
EMR-based nudges—alerts, reminders, or suggestions based on diagnostic data—guide clinicians toward evidence-based choices without restricting autonomy [15]. One study used a BPA to remind providers that positive urine cultures without urinary symptoms don’t require treatment, thereby decreasing antimicrobial utilization from 6.3 to 2.2 days per patient [4].
In extreme cases, complete restriction of urine culture results for non-catheterized patients has shown promise. This approach reduced antimicrobial prescribing for asymptomatic bacteriuria by 36% in one study [4].
Table: Summary of Interventions by Phase and Impact
| Phase | Intervention | Example Application | Documented Impact |
| Ordering | Hard stops | C. difficile testing | 33% reduction in test ordering [4] |
| Ordering | Provider-only ordering | Blood cultures | Reduction from 224.3 to 99.7 cultures per 1,000 patient-days [12] |
| Processing | Reflex testing | Urine cultures based on pyuria | 30% reduction in cultures performed [4] |
| Processing | Two-step algorithms | C. difficile testing | Reduction in false positive results [4] |
| Reporting | Cascade reporting | Antimicrobial susceptibilities | Decreased inappropriate prescribing [14] |
| Reporting | EMR nudges | Asymptomatic bacteriuria | Reduction from 6.3 to 2.2 days of antimicrobial use [4] |
Clearly, diagnostic stewardship interventions can produce measurable improvements across all testing phases, thereby reducing both diagnostic errors and unnecessary treatments.
Case Studies of Diagnostic Stewardship Reducing Errors in the ED 
Real-world implementations of diagnostic stewardship principles have demonstrated measurable reductions in medical errors in emergency settings. These case studies illustrate how structured interventions yield tangible improvements in clinical outcomes.
Urine culture reflex testing in ICU settings
A reflex urine culture protocol implemented across adult ICUs demonstrated remarkable effectiveness in reducing unnecessary testing. Using this approach, urinalysis was performed first, with cultures performed only when pyuria (urine WBC count >10 per high-power field) was present [16]. The results were compelling:
- 30% immediate decrease in urine culture rates post-intervention [16]
- 28% reduction in bacteriuria detection rates [16]
- The proportion of patients detected to have bacteriuria fell from 31% pre-intervention to 16% post-intervention [16]
Most notably, the proportion of patients who started new antimicrobials solely based on urine culture results decreased from 41% to 23% [16]. Hence, reflex testing effectively reduced both unnecessary diagnostics and treatments.
CDI test ordering restrictions and antimicrobial reduction
A healthcare system-wide EMR intervention aimed at decreasing fluoroquinolone (FQ) use produced substantial benefits. The system removed standalone FQ orders and replaced them with order sets that include built-in clinical decision support [2]. This intervention:
First, caused an immediate decrease in inpatient FQ use beyond the pre-existing downward trend [2]. Second, while hospital-onset CDI rates remained unchanged, post-discharge CDI incidence shifted from a stable rate to a steady decrease of 2.5% per month [2].
Blood culture checklist implementation in pediatric ICUs
The BrighT STAR collaborative implemented blood culture diagnostic stewardship across 14 pediatric ICUs nationwide from 2017-2021 [17]. Using a checklist-style protocol, the intervention yielded:
- 33% reduction in overall blood culture rates [17]
- 13% reduction in broad-spectrum antibiotic use [17]
- No changes in safety balancing measures [17]
Similar results emerged from earlier studies, with one pediatric ICU demonstrating a nearly 50% reduction in blood cultures without increased mortality or missed sepsis [18].
Machine learning model for blood culture prediction
Recent advances employ machine learning to identify patients at low risk for bacteremia. In one study, a gradient-boosted tree model achieved an Area Under the ROC curve of 0.77, correctly identifying 69% of blood cultures as likely negative with a negative predictive value of> 94% [19]. In a real-time prospective evaluation, this model maintained its performance, enabling clinicians to safely withhold blood culture analyses in at least 30% of ED patients [20].
Implementation Challenges and Guidelines for ED Settings
Successful implementation of diagnostic stewardship in busy emergency departments requires overcoming unique operational hurdles through systematic approaches. These challenges demand thoughtful solutions that balance clinical effectiveness with practicality in the real world.
Diagnostic stewardship guidelines and institutional support
Effective diagnostic stewardship programs necessitate visible leadership commitment and administrative backing. Studies show that clear leadership support markedly improves frontline provider acceptance of diagnostic interventions [7]. The ideal program structure requires:
- Physician champions with institutional backing
- Multidisciplinary engagement, including pharmacists, nurses, and infection prevention staff
- Integration with existing laboratory utilization committees [7]
Avoiding alert fatigue in EMR systems
Excessive electronic alerts rapidly diminish effectiveness through provider desensitization. Studies indicate that low alert acceptance often stems from poor targeting, incorrect information, or un-actionable guidance [21]. Therefore, interruptive clinical decision support systems must be used sparingly and monitored closely [22]. User-centered EMR design facilitates appropriate test ordering by presenting contextual information—such as showing recent laxative use when ordering C. difficile testing [4].
Engaging microbiology labs and AMS teams
Microbiology laboratories serve as crucial partners in diagnostic stewardship initiatives. Their timely provision of accurate results directly impacts clinical outcomes and optimal antibiotic use [23]. Laboratory staff can present data in ways that support diagnostic excellence [24], yet actively participating in antimicrobial stewardship committees remains their most valuable contribution [23].
Training ED clinicians on diagnostic stewardship principles
Traditional educational approaches alone rarely produce lasting practice changes [8]. Thus, education is most effective when combined with audit-and-feedback mechanisms and behavioral nudges targeting specific barriers [8]. Focused training can dramatically improve understanding—a single 90-minute session increased statistical literacy from 50% to 90% among medical trainees [25].

Conclusion 

Diagnostic stewardship offers a powerful framework for reducing medical errors in emergency department settings. Throughout this article, we examined how systematic approaches to test ordering, processing, and interpretation directly impact clinical outcomes and patient safety. Evidence clearly demonstrates that proper diagnostic stewardship not only reduces unnecessary testing but also decreases inappropriate antimicrobial use and shortens hospital stays.
The benefits extend beyond individual patients to healthcare systems as a whole. Consider these key takeaways:
- History and physical examination provide 73%-94% of necessary diagnostic information, reinforcing the value of clinical judgment • Implementation of reflex testing protocols can reduce unnecessary cultures by up to 30% • Hard stops and soft stops in electronic ordering systems decrease inappropriate test utilization by 23-33% • Machine learning models now achieve >94% negative predictive value for identifying low-risk bacteremia cases
Despite these promising results, challenges remain. Alert fatigue threatens the effectiveness of electronic interventions, while successful implementation requires sustained institutional commitment. Therefore, diagnostic stewardship must evolve from isolated interventions to comprehensive programs with multidisciplinary involvement.
Looking ahead, the future of diagnostic excellence depends on our ability to balance technology with clinical expertise. Educational initiatives must accompany system-level changes, empowering clinicians to make evidence-based decisions about test ordering and interpretation. Additionally, ongoing collaboration between emergency departments, microbiology laboratories, and antimicrobial stewardship teams will strengthen these efforts.
Though diagnostic stewardship originated in infection prevention, its principles apply broadly across emergency medicine. As testing technology advances, the need for thoughtful stewardship grows proportionally. Ultimately, diagnostic stewardship represents not merely a quality improvement initiative but a fundamental shift in how we approach diagnostic testing—prioritizing accuracy, appropriateness, and patient outcomes above all else.
Frequently Asked Questions: 
FAQs
Q1. What is diagnostic stewardship, and how does it differ from antimicrobial stewardship? Diagnostic stewardship is a systematic approach to ensure appropriate test ordering, interpretation, and action on results. It focuses on whether antibiotics are needed based on accurate diagnosis, while antimicrobial stewardship primarily addresses how antibiotics are prescribed once a diagnosis is made.
Q2. How can diagnostic stewardship reduce medical errors in emergency departments? Diagnostic stewardship can reduce errors by implementing interventions across the testing process, including clinical decision support tools, reflex testing protocols, and selective reporting of results. These measures help prevent unnecessary testing, reduce false positives, and guide appropriate treatment decisions.
Q3. What are some common diagnostic errors in emergency departments? Common diagnostic errors in EDs include overdiagnosis of asymptomatic bacteriuria, false-positive Clostridioides difficile infections, contaminated blood cultures leading to unnecessary treatment, and misdiagnosis of ventilator-associated pneumonia.
Q4. Can you provide an example of a successful diagnostic stewardship intervention? One successful intervention involved implementing a reflex urine culture protocol in adult ICUs. This approach resulted in a 30% decrease in urine culture rates, a 28% reduction in bacteriuria detection, and a significant decrease in unnecessary antimicrobial prescriptions based solely on urine culture results.
Q5. What challenges exist in implementing diagnostic stewardship programs in emergency departments? Key challenges include avoiding alert fatigue in EMR systems, engaging microbiology labs and antimicrobial stewardship teams, and effectively training ED clinicians on diagnostic stewardship principles. Successful implementation requires visible leadership commitment, multidisciplinary engagement, and a balance between technology and clinical expertise.
References: 
[1] – https://emcrit.org/ibcc/vap/
[2] – https://pmc.ncbi.nlm.nih.gov/articles/PMC9495417/
[3] – https://www.cdc.gov/antibiotic-use/core-elements/pdfs/fs-bloodculture-508.pdf
[4] – https://pmc.ncbi.nlm.nih.gov/articles/PMC9949764/
[5] – https://journals.asm.org/doi/10.1128/jcm.00941-24
[6] – https://www.ncbi.nlm.nih.gov/books/NBK588118/
[9] – https://www.sciencedirect.com/science/article/abs/pii/S0196655322007830
[10] – https://pmc.ncbi.nlm.nih.gov/articles/PMC3623801/
[12] – https://pmc.ncbi.nlm.nih.gov/articles/PMC12509148/
[13] – https://www.testingwisely.com/diagnostic-stewardship
[16] – https://pmc.ncbi.nlm.nih.gov/articles/PMC6342270/
[17] – https://pmc.ncbi.nlm.nih.gov/articles/PMC11428017/
[18] – https://www.eurekalert.org/news-releases/718775
[19] – https://pubmed.ncbi.nlm.nih.gov/34983764/
[20] – https://www.sciencedirect.com/science/article/pii/S2352396422003577
[21] – https://pmc.ncbi.nlm.nih.gov/articles/PMC9132737/
[22] – https://www.thieme-connect.com/products/ejournals/pdf/10.1055/s-0042-1748856.pdf
[23] – https://pmc.ncbi.nlm.nih.gov/articles/PMC5217798/
[24] – https://www.cdc.gov/patient-safety/hcp/hospital-dx-excellence/index.html
Video Section
Check out our extensive video library (see channel for our latest videos)
Recent Articles


