The Cancer Risk Paradox of Anti-Aging Therapies

Abstract
The relationship between anti-aging interventions and cancer risk presents a complex biological paradox that demands careful examination by healthcare practitioners. While many anti-aging therapies aim to promote cellular health and longevity, they may inadvertently create conditions that favor malignant transformation and tumor progression. This paper examines the mechanisms underlying this paradox, analyzing how interventions targeting cellular senescence, telomere extension, growth factor modulation, and stem cell activation can simultaneously offer anti-aging benefits while potentially increasing cancer susceptibility. Through examination of current research, clinical observations, and mechanistic studies, this analysis reveals the delicate balance between promoting healthy aging and maintaining cancer surveillance systems. The evidence suggests that many pathways involved in aging and cancer prevention overlap in ways that create inherent trade-offs. Healthcare providers must understand these relationships to make informed decisions about anti-aging interventions and provide appropriate patient counseling. This review synthesizes current knowledge about specific therapies, their mechanisms of action, and associated cancer risks, providing practical guidance for clinical decision-making in the emerging field of longevity medicine.
Recent articles. Check out our extensive video library.
Introduction
The pursuit of healthy aging has led to rapid development of therapies targeting fundamental aging processes. These interventions range from pharmaceutical compounds to lifestyle modifications, each designed to slow or reverse age-related decline. However, mounting evidence suggests that many anti-aging strategies may inadvertently increase cancer risk through shared biological pathways.
The aging process and cancer development share common molecular mechanisms that create inherent conflicts between longevity promotion and cancer prevention. Understanding these relationships has become critical as anti-aging therapies gain popularity and enter clinical practice. The paradox emerges from the fact that many cellular processes that drive aging also serve as natural cancer protection mechanisms.
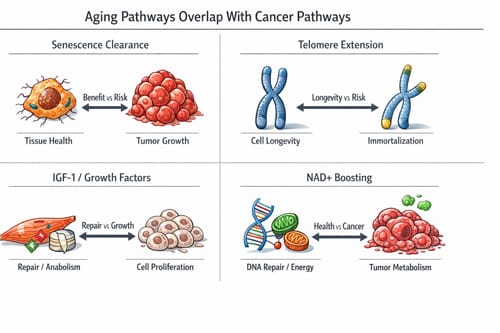
Cellular senescence, often viewed as a hallmark of aging, actually functions as a powerful tumor suppressor mechanism. When cells detect DNA damage or oncogenic stress, they enter senescence to prevent malignant transformation. Anti-aging therapies that eliminate senescent cells or prevent senescence may remove this protective barrier. Similarly, interventions that stimulate cell division, enhance growth factor signaling, or extend cellular lifespan can inadvertently promote conditions favorable to cancer development.
This paradox extends beyond theoretical concerns. Clinical observations and research studies have documented increased cancer incidence associated with certain anti-aging approaches. Growth hormone therapy, testosterone replacement, and some dietary supplements have shown associations with various malignancies. The challenge for healthcare providers lies in weighing potential longevity benefits against cancer risks while making evidence-based treatment recommendations.
The complexity of this relationship demands careful analysis of specific mechanisms, individual risk factors, and available evidence. Each anti-aging intervention carries unique risk-benefit profiles that must be evaluated within the context of patient-specific factors including age, genetic background, medical history, and overall health status.
Fundamental Mechanisms of the Aging-Cancer Paradox
Cellular Senescence and Tumor Suppression
Cellular senescence represents one of the most important examples of the aging-cancer paradox. Senescent cells accumulate with age and contribute to tissue dysfunction, inflammation, and age-related pathologies. These cells secrete inflammatory factors, growth factors, and matrix-degrading enzymes that create a pro-aging tissue environment. Consequently, senescent cell elimination has emerged as a promising anti-aging strategy.
However, senescence serves as a critical tumor suppressor mechanism. When cells experience DNA damage, oncogene activation, or other stressful stimuli, they may enter permanent growth arrest rather than becoming malignant. This response prevents damaged cells from proliferating and forming tumors. The senescence program includes activation of tumor suppressor pathways, particularly p53 and p16, which halt cell division and maintain genomic stability.
Anti-aging therapies targeting senescence face the challenge of removing beneficial tumor suppression while addressing age-related dysfunction. Senolytic drugs that selectively eliminate senescent cells must carefully balance these competing effects. Research has shown that senescent cell clearance can improve healthspan and reduce age-related pathologies, but the long-term cancer implications remain under investigation.
The timing and context of senolytic interventions appear crucial. Early-life senescent cell accumulation may primarily serve tumor suppressor functions, while late-life senescence may contribute more to aging pathologies. This age-dependent shift in senescence function complicates therapeutic targeting and risk assessment.
Telomere Biology and Cancer Risk
Telomere extension represents another area where anti-aging benefits conflict with cancer protection. Telomeres protect chromosome ends and shorten with each cell division, eventually triggering senescence or cell death. Short telomeres contribute to aging by limiting cellular replicative capacity and promoting genomic instability.
Telomerase activation can extend telomeres and potentially slow cellular aging. Some anti-aging interventions aim to boost telomerase activity or protect telomeres from shortening. While this approach may enhance cellular longevity, it also removes a natural barrier to unlimited cell division that normally constrains cancer development.
Cancer cells frequently reactivate telomerase to achieve immortalization, allowing unlimited proliferative potential. Approximately 90% of human cancers show telomerase reactivation, highlighting its importance in malignant transformation. Therapeutic telomerase activation for anti-aging purposes could theoretically provide similar benefits to pre-cancerous or early cancer cells.
The relationship between telomere length and cancer risk varies by cancer type and individual circumstances. Some studies suggest that very short telomeres increase certain cancer risks through genomic instability, while moderately short telomeres may provide protection by limiting cellular proliferation. This complex relationship makes telomere-targeted anti-aging therapies particularly challenging to implement safely.
Growth Factor Signaling Pathways
Many anti-aging interventions target growth factor signaling pathways that regulate cellular metabolism, protein synthesis, and stress responses. The insulin-like growth factor (IGF-1) pathway, growth hormone signaling, and mechanistic target of rapamycin (mTOR) pathway have all been implicated in aging processes and represent targets for longevity interventions.
These same pathways play important roles in cancer development and progression. IGF-1 promotes cell survival, proliferation, and angiogenesis while inhibiting apoptosis. Elevated IGF-1 levels have been associated with increased risk of several cancer types, including prostate, breast, and colorectal cancers. Growth hormone therapy, sometimes used for anti-aging purposes, can increase IGF-1 levels and potentially elevate cancer risk.
The mTOR pathway integrates nutrient sensing, energy status, and growth signals to control cell growth and division. While mTOR inhibition has shown anti-aging effects in laboratory studies, the pathway’s role in cancer is complex. mTOR activation can promote tumor growth and progression, but mTOR inhibitors are also used as cancer treatments. This dual role creates uncertainty about optimal targeting strategies for anti-aging purposes.
Growth factor modulation for anti-aging must carefully consider cancer implications. Interventions that enhance anabolic signaling may promote healthy aging in some contexts while creating conditions favorable to cancer development in others. Individual risk assessment becomes critical when considering growth factor-targeted therapies.

Specific Anti-Aging Therapies and Cancer Risks 
Hormone Replacement Therapies
Hormone replacement represents one of the most established areas where anti-aging benefits intersect with cancer concerns. Declining hormone levels contribute to many aging-related changes, making hormone replacement an attractive anti-aging strategy. However, several hormones have well-documented cancer associations that complicate their use.
Estrogen replacement therapy provides relief from menopausal symptoms and may offer bone and cardiovascular benefits. However, estrogen stimulates proliferation of hormone-sensitive tissues and increases risks of breast and endometrial cancers. The Women’s Health Initiative study demonstrated increased breast cancer risk with combined estrogen-progestin therapy, fundamentally changing hormone replacement practices.
Testosterone replacement therapy has gained popularity for treating age-related testosterone decline in men. While testosterone can improve muscle mass, bone density, and quality of life, concerns exist about prostate cancer risk. Testosterone stimulates prostate cell growth and may accelerate existing prostate cancers. Although epidemiological evidence for increased prostate cancer incidence remains mixed, the biological rationale for concern persists.
Growth hormone therapy represents another hormone intervention with anti-aging appeal but cancer concerns. Growth hormone levels decline with age, contributing to decreased muscle mass, increased fat accumulation, and reduced exercise capacity. Growth hormone replacement can reverse some of these changes but may increase cancer risk through IGF-1 stimulation and enhanced cell proliferation.
Caloric Restriction Mimetics
Caloric restriction has demonstrated anti-aging effects across multiple species, leading to interest in compounds that mimic these benefits without requiring dietary restriction. Resveratrol, metformin, and rapamycin represent prominent examples of caloric restriction mimetics with potential anti-aging properties.
Resveratrol activates sirtuin proteins involved in cellular stress responses and metabolic regulation. While preclinical studies suggest anti-aging and anti-cancer properties, human evidence remains limited. Some studies indicate that resveratrol may have both cancer-protective and cancer-promoting effects depending on dose, timing, and cellular context.
Metformin, a diabetes medication, has shown associations with reduced cancer incidence and improved longevity in epidemiological studies. The drug activates AMP-activated protein kinase (AMPK) and inhibits mTOR signaling, potentially providing anti-aging benefits. Unlike many anti-aging interventions, metformin appears to reduce rather than increase cancer risk, making it an attractive option for further investigation.
Rapamycin inhibits mTOR signaling and extends lifespan in laboratory animals. The drug has established anti-cancer properties as an approved cancer treatment, suggesting favorable cancer risk profiles for anti-aging applications. However, rapamycin also suppresses immune function, potentially reducing cancer surveillance and increasing infection risks.
Stem Cell Therapies
Stem cell interventions aim to replace aged or damaged cells with fresh, functional cells capable of tissue regeneration. These approaches range from stem cell transplantation to interventions that activate endogenous stem cell populations. While stem cell therapies offer promise for tissue repair and regeneration, they also raise cancer concerns.
Stem cells possess many characteristics that, when dysregulated, contribute to cancer development. These include self-renewal capacity, resistance to cell death, and ability to differentiate into multiple cell types. Cancer stem cells share many properties with normal stem cells and may arise from stem cell transformation or dedifferentiation of mature cells.
Embryonic stem cells and induced pluripotent stem cells carry particular cancer risks due to their unlimited proliferative potential and pluripotent characteristics. These cells can form teratomas when transplanted and may undergo malignant transformation. Clinical applications require careful protocols to ensure complete differentiation and eliminate undifferentiated cells before transplantation.
Adult stem cell therapies generally carry lower cancer risks but are not without concerns. Mesenchymal stem cells, commonly used in regenerative medicine, have shown variable effects on cancer development. Some studies suggest tumor-promoting effects through growth factor secretion and immune suppression, while others indicate anti-tumor properties.
Table 1: Anti-Aging Therapies and Associated Cancer Risks
|
Therapy Category |
Specific Examples |
Proposed Anti-Aging Mechanism |
Cancer Risk Concerns |
Risk Level |
|---|---|---|---|---|
|
Hormone Replacement |
Estrogen, Testosterone, Growth Hormone |
Restore declining hormone levels |
Stimulation of hormone-sensitive cancers |
High |
|
Senolytic Drugs |
Dasatinib + Quercetin, Fisetin |
Eliminate senescent cells |
Removal of tumor suppressor mechanisms |
Moderate |
|
Telomerase Activators |
TA-65, Astragalus extracts |
Extend cellular lifespan |
Enable unlimited cell division |
High |
|
Growth Factor Modulators |
IGF-1, Growth Hormone |
Enhance anabolic signaling |
Promote cell proliferation and survival |
Moderate-High |
|
Caloric Restriction Mimetics |
Resveratrol, Metformin, Rapamycin |
Activate longevity pathways |
Variable, context-dependent |
Low-Moderate |
|
Stem Cell Therapies |
MSCs, iPSCs, ESCs |
Tissue regeneration and repair |
Transformation and tumor formation |
Moderate-High |
|
NAD+ Boosters |
NMN, NR, Niacin |
Enhance cellular energy metabolism |
Fuel cancer cell metabolism |
Low-Moderate |
NAD+ Precursors and Metabolic Modulators
Nicotinamide adenine dinucleotide (NAD+) levels decline with age, contributing to cellular energy dysfunction and age-related pathologies. NAD+ precursors such as nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR) have gained attention as potential anti-aging supplements that can boost cellular NAD+ levels.
While NAD+ enhancement may provide anti-aging benefits through improved mitochondrial function and DNA repair, concerns exist about potential cancer promotion. Cancer cells have high energy demands and may benefit from enhanced NAD+ availability. Some studies suggest that NAD+ boosting could accelerate tumor growth by providing metabolic fuel for rapidly dividing cancer cells.
However, the relationship between NAD+ and cancer is complex. NAD+ also supports DNA repair mechanisms and cellular stress responses that may help prevent cancer development. The net effect of NAD+ modulation on cancer risk likely depends on individual circumstances, including existing cancer burden and genetic factors.
Other metabolic modulators targeting mitochondrial function, cellular energy production, and oxidative stress may face similar paradoxes. While these interventions may improve cellular health and longevity, they could also provide benefits to cancer cells that have similar metabolic needs.

Clinical Applications and Risk Assessment
Patient Selection and Risk Stratification
Implementing anti-aging therapies requires careful patient selection and risk stratification to optimize benefit-risk ratios. Healthcare providers must evaluate individual cancer risk factors, including family history, genetic predisposition, previous cancer diagnoses, and current health status when considering anti-aging interventions.
Patients with strong family histories of cancer or known genetic predispositions may face elevated risks from certain anti-aging therapies. For example, individuals with BRCA mutations who have increased breast cancer risk should approach estrogen-containing interventions with particular caution. Similarly, men with family histories of prostate cancer may need careful consideration before beginning testosterone replacement therapy.
Age represents another important factor in risk-benefit calculations. Younger individuals may face greater long-term cancer risks from anti-aging interventions due to longer exposure periods and more active cellular division rates. Conversely, older patients may have shorter time horizons for cancer development and greater immediate needs for anti-aging benefits.
Existing health conditions also influence risk assessments. Patients with diabetes may benefit more from metformin-based interventions due to established therapeutic benefits beyond anti-aging effects. Those with cardiovascular disease may prioritize interventions with proven cardiovascular benefits even if cancer risks exist.
Monitoring and Surveillance Strategies
Patients receiving anti-aging therapies with potential cancer risks require enhanced surveillance and monitoring protocols. Regular cancer screening becomes more important when using interventions that may increase malignancy risks or accelerate existing cancer progression.
Hormone replacement therapy recipients need regular mammograms, pelvic examinations, and other gender-specific cancer screenings. Men receiving testosterone therapy should undergo regular prostate examinations and prostate-specific antigen monitoring. Growth hormone therapy recipients may benefit from broader cancer surveillance due to the hormone’s effects on multiple organ systems.
Biomarker monitoring can help detect early signs of cancer development or progression. Regular measurement of tumor markers, inflammatory indicators, and growth factor levels may provide early warning signs of potential problems. However, the predictive value of many biomarkers remains limited, and surveillance strategies must balance detection benefits with costs and patient burden.
Laboratory monitoring should also assess therapy effectiveness and safety parameters beyond cancer risk. Hormone levels, metabolic markers, and organ function tests help ensure that interventions provide intended benefits while avoiding non-cancer adverse effects.
Combination Therapy Considerations
Many patients seek multiple anti-aging interventions simultaneously, creating complex interaction patterns that may modify cancer risks. Combination therapies may have additive, synergistic, or antagonistic effects on cancer development that differ from individual intervention risks.
For example, combining hormone replacement with growth factor stimulation may create greater cancer risks than either intervention alone. Conversely, including interventions with anti-cancer properties, such as metformin or rapamycin, in anti-aging regimens may help offset cancer risks from other components.
Drug interactions and metabolic effects of combination therapies require careful consideration. Some anti-aging interventions may alter metabolism or clearance of others, potentially increasing exposure levels and associated risks. Healthcare providers must understand these interactions when designing treatment regimens.
The sequence and timing of interventions may also influence cancer risks. Sequential rather than simultaneous introduction allows for individual risk assessment and may help identify problems before they become serious. This approach also permits discontinuation of problematic interventions while continuing beneficial ones.
Comparison with Conventional Medical Approaches 
Traditional Cancer Prevention Strategies
Conventional cancer prevention focuses on risk factor modification, screening, and chemoprevention in high-risk populations. These approaches generally aim to reduce cancer incidence through lifestyle modifications, environmental exposure reduction, and early detection rather than promoting longevity through biological interventions.
Traditional prevention strategies typically carry lower risks of inadvertent cancer promotion because they do not directly manipulate cellular growth and survival pathways. Smoking cessation, dietary modifications, exercise programs, and sun protection reduce cancer risks without the biological complexity of anti-aging therapies.
However, conventional prevention approaches may have limited effects on aging processes beyond their cancer prevention benefits. While healthy lifestyle choices promote overall health and may slow some aging processes, they do not directly target fundamental aging mechanisms in the way that biological anti-aging interventions attempt to do.
The integration of anti-aging therapies with conventional cancer prevention strategies may provide optimal outcomes. Patients pursuing anti-aging interventions should maintain or intensify conventional cancer prevention measures to help offset any increased risks from longevity therapies.
Established Medical Therapies with Dual Effects
Some established medical therapies demonstrate both anti-aging and cancer prevention properties, providing examples of interventions that avoid the typical aging-cancer paradox. Metformin represents the best-studied example, with documented benefits for diabetes treatment, potential anti-aging effects, and reduced cancer incidence.
Statins, primarily used for cholesterol reduction and cardiovascular disease prevention, have shown associations with reduced cancer risks and potential anti-aging effects through anti-inflammatory mechanisms. These drugs demonstrate that some interventions can simultaneously address aging and cancer concerns.
Aspirin provides another example of a medication with multiple benefits that may include both longevity promotion and cancer prevention. Low-dose aspirin reduces cardiovascular events and has shown cancer prevention benefits, particularly for colorectal cancer, while potentially contributing to healthy aging through anti-inflammatory effects.
These examples suggest that the aging-cancer paradox is not inevitable and that careful intervention selection can avoid conflicting effects. However, such interventions remain relatively rare, and most anti-aging therapies continue to present cancer-related trade-offs.

Challenges and Limitations
Research and Evidence Gaps
The field of anti-aging medicine faces substantial evidence gaps that complicate risk-benefit assessments for cancer concerns. Most anti-aging interventions lack long-term human studies that would be necessary to fully characterize cancer risks. Laboratory studies and short-term clinical trials provide limited insight into long-term cancer outcomes.
The complexity of aging and cancer biology makes it difficult to predict intervention effects from mechanistic understanding alone. Biological systems have redundant pathways, feedback mechanisms, and individual variations that can modify expected outcomes. What appears beneficial or harmful in controlled laboratory settings may behave differently in human populations.
Cancer development occurs over years to decades, making it difficult to assess cancer risks from interventions within typical clinical trial timeframes. Many anti-aging therapies have been available for only short periods, insufficient for cancer outcome evaluation. Long-term observational studies will be necessary but take time to generate meaningful results.
Individual genetic and environmental factors likely modify cancer risks from anti-aging interventions, but personalized risk prediction remains limited. Genetic testing can identify some high-risk individuals, but the majority of cancer risk factors remain unknown or poorly quantified.
Regulatory and Safety Considerations
The regulatory landscape for anti-aging interventions remains complex and evolving. Many compounds marketed for anti-aging purposes exist in regulatory gray areas, available as dietary supplements or through off-label prescribing without specific approval for longevity indications.
This regulatory uncertainty creates challenges for healthcare providers trying to make evidence-based recommendations. Without formal regulatory review, safety and efficacy data may be limited, and quality control standards may vary. Patients may access interventions through non-medical channels that lack professional oversight.
The lack of standardized protocols for anti-aging interventions makes it difficult to compare outcomes and risks across different approaches. Dosing, timing, monitoring, and combination strategies vary widely, creating inconsistent risk profiles that complicate clinical decision-making.
Professional medical organizations have yet to develop comprehensive guidelines for anti-aging medicine that adequately address cancer risk concerns. This guidance gap leaves individual practitioners to make complex risk-benefit decisions without institutional support or standardized approaches.
Ethical and Informed Consent Issues
The uncertain risk-benefit profiles of many anti-aging interventions raise ethical questions about appropriate patient counseling and informed consent. Healthcare providers must communicate complex and uncertain risks while acknowledging limitations in current knowledge.
The desire for longevity and health improvement may influence patient decision-making in ways that minimize perceived risks or overestimate potential benefits. Providers must ensure that patients understand both known risks and uncertainties while respecting patient autonomy in treatment choices.
The marketing of anti-aging interventions often emphasizes benefits while minimizing risks, potentially creating unrealistic patient expectations. Healthcare providers may need to counter marketing messages with balanced, evidence-based information about cancer risks and other concerns.
Questions exist about appropriate patient populations for anti-aging interventions given uncertain risk-benefit profiles. Should these therapies be reserved for patients with established age-related diseases, or are they appropriate for healthy individuals seeking longevity enhancement? These decisions involve value judgments about acceptable risk levels that may vary among individuals and healthcare providers.
Future Research Directions
Biomarker Development and Risk Prediction
Future research should focus on developing biomarkers that can predict individual responses to anti-aging interventions and associated cancer risks. Genetic markers, protein signatures, and metabolic profiles may help identify patients most likely to benefit from specific interventions while avoiding those at highest risk for adverse outcomes.
Circulating tumor markers and other early cancer detection methods may enable closer monitoring of patients receiving potentially risky anti-aging interventions. Improved detection capabilities could allow earlier intervention if cancer development occurs, potentially mitigating long-term risks.
Longitudinal studies tracking biomarker changes in patients receiving anti-aging therapies could provide insights into intervention effects and help optimize dosing and monitoring strategies. These studies should include both anti-aging outcomes and cancer development endpoints.
Research into the timing and reversibility of intervention effects may inform strategies for minimizing cancer risks while maximizing anti-aging benefits. Understanding whether intervention effects persist after discontinuation could enable intermittent dosing strategies that reduce cumulative cancer risks.
Mechanism-Based Intervention Design
Future anti-aging interventions should be designed with explicit consideration of cancer risk mechanisms from the earliest development stages. Rather than retrofitting cancer risk assessments to existing interventions, new therapies should target aging processes while preserving or enhancing cancer protection mechanisms.
Research into pathway-specific interventions may enable more targeted approaches that avoid broad effects on cancer-related processes. For example, interventions that target specific aspects of cellular senescence while preserving tumor suppressor functions may offer better risk-benefit profiles.
Combination therapy research should explore whether pairing anti-aging interventions with cancer protective compounds can provide net benefits. Rational combination design based on mechanistic understanding may overcome individual intervention limitations.
The development of reversible or controllable interventions may provide safety advantages by enabling rapid discontinuation if cancer concerns arise. Drug delivery systems, temporary interventions, and feedback-controlled therapies represent potential approaches to this challenge.
Personalized Medicine Approaches
The future of anti-aging medicine likely requires personalized approaches that account for individual genetic, environmental, and medical factors in intervention selection and risk assessment. Genetic testing, biomarker profiling, and risk modeling may enable more precise treatment recommendations.
Research into genetic modifiers of intervention effects could identify patient subgroups with favorable risk-benefit profiles for specific therapies. For example, individuals with particular genetic variants might benefit from certain interventions while others face unacceptable risks.
Artificial intelligence and machine learning approaches may help integrate complex patient data to predict intervention outcomes and optimize treatment selections. These tools could incorporate genetic information, medical history, biomarker data, and other factors to generate personalized risk-benefit assessments.
Clinical decision support systems incorporating cancer risk predictions could help healthcare providers make more informed recommendations about anti-aging interventions while ensuring appropriate patient counseling about potential risks and benefits.
Conclusion 

The cancer risk paradox of anti-aging therapies represents one of the most important challenges facing the emerging field of longevity medicine. While many interventions targeting aging processes offer promise for improving healthspan and extending healthy longevity, they may simultaneously increase cancer risks through shared biological pathways between aging and cancer development.
This paradox is not merely theoretical but has practical implications for patient care and clinical decision-making. Healthcare providers must carefully weigh potential longevity benefits against cancer risks while acknowledging substantial uncertainties in current knowledge. The evidence suggests that many popular anti-aging interventions, including hormone replacement therapies, senolytic drugs, and growth factor modulators, may increase cancer risks through mechanisms that are intimately connected to their anti-aging effects.
However, the relationship between aging interventions and cancer risk is not uniform across all therapies. Some interventions, such as metformin and rapamycin, may provide anti-aging benefits while simultaneously reducing cancer risks. These examples suggest that the aging-cancer paradox is not inevitable and that careful intervention selection and design may overcome these challenges.
The path forward requires continued research into the mechanisms underlying aging-cancer relationships, development of better risk prediction tools, and creation of interventions that can target aging processes while preserving cancer protection mechanisms. Healthcare providers must maintain awareness of these complex relationships while providing balanced counseling to patients considering anti-aging interventions.
Patient selection and monitoring strategies should account for individual cancer risk factors, and enhanced surveillance may be appropriate for patients receiving interventions with known or suspected cancer risks. The integration of anti-aging therapies with conventional cancer prevention strategies may provide optimal outcomes for many patients.
Key Takeaways
The relationship between anti-aging therapies and cancer risk involves fundamental biological trade-offs that cannot be ignored. Many cellular processes that contribute to aging also serve important tumor suppressor functions, creating inherent conflicts between longevity promotion and cancer prevention.
Healthcare providers should approach anti-aging interventions with careful consideration of individual patient factors, including cancer risk factors, age, health status, and personal preferences. Enhanced surveillance and monitoring may be appropriate for patients receiving interventions with potential cancer risks.
The evidence base for long-term cancer risks from most anti-aging interventions remains limited due to the relatively recent emergence of these therapies and the long timeframe required for cancer development. This uncertainty should be clearly communicated to patients considering these treatments.
Some anti-aging interventions appear to have more favorable cancer risk profiles than others. Metformin, rapamycin, and certain lifestyle interventions may provide anti-aging benefits while reducing rather than increasing cancer risks.
Future research should focus on developing personalized approaches to anti-aging medicine that account for individual genetic and medical factors in treatment selection and risk assessment. The integration of biomarker development, mechanism-based intervention design, and personalized medicine approaches offers promise for overcoming current limitations.
Frequently Asked Questions: 
What is the cancer risk paradox in anti-aging medicine?
The cancer risk paradox refers to the observation that many anti-aging therapies may inadvertently increase cancer risk through the same mechanisms that provide anti-aging benefits. This occurs because many biological processes involved in aging also serve as natural cancer protection mechanisms.
Which anti-aging therapies carry the highest cancer risks?
Hormone replacement therapies, particularly estrogen and testosterone, carry well-documented cancer risks. Growth hormone therapy and interventions that target telomerase activation or eliminate cellular senescence also present substantial theoretical cancer risks based on their mechanisms of action.
Are there any anti-aging interventions that may actually reduce cancer risk?
Yes, some interventions appear to provide both anti-aging benefits and cancer protection. Metformin has shown associations with reduced cancer incidence in addition to potential longevity benefits. Rapamycin, while primarily studied as a cancer treatment, also demonstrates anti-aging properties in laboratory studies.
How should patients with family histories of cancer approach anti-aging therapies?
Patients with strong family histories of cancer or known genetic predispositions should exercise particular caution with anti-aging interventions. They should work closely with healthcare providers to assess individual risk factors and may benefit from enhanced cancer surveillance if they choose to proceed with potentially risky interventions.
What monitoring is recommended for patients receiving anti-aging therapies?
Monitoring strategies should be tailored to specific interventions and individual risk factors. Generally, enhanced cancer screening appropriate for age and gender is recommended. Specific monitoring for hormone-related therapies includes regular examinations of hormone-sensitive organs and relevant biomarker testing.
Can anti-aging interventions be safely combined with conventional cancer prevention strategies?
Yes, combining anti-aging interventions with conventional cancer prevention strategies is generally recommended and may help offset potential cancer risks. This includes maintaining healthy lifestyle choices, appropriate cancer screening, and following established prevention guidelines.
How long do patients need to be monitored for cancer risks after starting anti-aging therapies?
Cancer development typically occurs over years to decades, so long-term monitoring is generally recommended. The specific duration depends on the intervention, individual risk factors, and emerging research about long-term effects. Patients should maintain regular follow-up care indefinitely.
Are dietary supplements marketed for anti-aging safer than prescription therapies?
Not necessarily. Many dietary supplements targeting aging processes work through similar biological mechanisms as prescription therapies and may carry similar cancer risk concerns. Additionally, supplements often lack the rigorous testing and quality control standards applied to prescription medications.
Should younger people avoid anti-aging therapies due to cancer concerns?
Younger individuals may face greater long-term cancer risks due to longer potential exposure periods. However, individual circumstances vary, and some younger patients with specific medical conditions may benefit from certain interventions. Age should be considered as part of overall risk-benefit assessment rather than an absolute contraindication.
What should patients do if they develop cancer while receiving anti-aging therapy?
Patients who develop cancer while receiving anti-aging therapy should immediately consult with both their prescribing physician and oncologist to determine whether the intervention should be discontinued or modified. The relationship between the therapy and cancer development should be evaluated, and treatment plans should be adjusted accordingly.
References: 
Adams, P. D., Jasper, H., & Rudolph, K. L. (2015). Aging-induced stem cell mutations as drivers for disease and cancer. Cell Stem Cell, 16(6), 601-612.
Blagosklonny, M. V. (2013). Aging is not programmed: Genetic pseudo-program is a shadow of developmental growth. Cell Cycle, 12(24), 3736-3742.
Campisi, J., Kapahi, P., Lithgow, G. J., Melov, S., Newman, J. C., & Verdin, E. (2019). From discoveries in ageing research to therapeutics for healthy ageing. Nature, 571(7764), 183-192.
Chang, J., Wang, Y., Shao, L., Laberge, R. M., Demaria, M., Campisi, J., … & Zhou, D. (2016). Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nature Medicine, 22(1), 78-83.
de Keizer, P. L. (2017). The fountain of youth by targeting senescent cells? Trends in Molecular Medicine, 23(1), 6-17.
Demaria, M., Ohtani, N., Youssef, S. A., Rodier, F., Toussaint, W., Mitchell, J. R., … & Campisi, J. (2014). An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Developmental Cell, 31(6), 722-733.
Fontana, L., Partridge, L., & Longo, V. D. (2010). Extending healthy life span–from yeast to humans. Science, 328(5976), 321-326.
Green, C. L., Lamming, D. W., & Fontana, L. (2022). Molecular mechanisms of dietary restriction promoting health and longevity. Nature Reviews Molecular Cell Biology, 23(1), 56-73.
Grosse, L., Wagner, N., Emelyanov, A., Molina, C., Lacas-Gervais, S., Wagner, K. D., & Bulavin, D. V. (2020). Defined p16High senescent cell types are indispensable for mouse healthspan. Cell Metabolism, 32(1), 87-99.
Harrison, D. E., Strong, R., Sharp, Z. D., Nelson, J. F., Astle, C. M., Flurkey, K., … & Miller, R. A. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature, 460(7253), 392-395.
Jaskelioff, M., Muller, F. L., Paik, J. H., Thomas, E., Jiang, S., Adams, A. C., … & DePinho, R. A. (2011). Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature, 469(7328), 102-106.
Justice, J. N., Nambiar, A. M., Tchkonia, T., LeBrasseur, N. K., Pascoe, R., Hashmi, S. K., … & Kirkland, J. L. (2019). Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine, 40, 554-563.
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2013). The hallmarks of aging. Cell, 153(6), 1194-1217.
Matheu, A., Maraver, A., Klatt, P., Flores, I., Garcia-Cao, I., Borras, C., … & Serrano, M. (2007). Delayed ageing through damage protection by the Arf/p53 pathway. Nature, 448(7151), 375-379.
Mosteiro, L., Pantoja, C., Alcazar, N., Marión, R. M., Chondronasiou, D., Rovira, M., … & Serrano, M. (2016). Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science, 354(6315), aaf4445.
Muñoz-Espín, D., & Serrano, M. (2014). Cellular senescence: From physiology to pathology. Nature Reviews Molecular Cell Biology, 15(7), 482-496.
Roichman, A., Kanfi, Y., Glazz, R., Naiman, S., Amit, U., Landa, N., … & Cohen, H. Y. (2017). SIRT6 overexpression improves various aspects of mouse healthspan. Journals of Gerontology Series A, 72(5), 603-615.
Rudolph, K. L., Chang, S., Lee, H. W., Blasco, M., Gottlieb, G. J., Greider, C., & DePinho, R. A. (1999). Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell, 96(5), 701-712.
Sharpless, N. E., & Sherr, C. J. (2015). Forging a signature of in vivo senescence. Nature Reviews Cancer, 15(7), 397-408.
Tchkonia, T., Zhu, Y., van Deursen, J., Campisi, J., & Kirkland, J. L. (2013). Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. Journal of Clinical Investigation, 123(3), 966-972.
van Deursen, J. M. (2014). The role of senescent cells in ageing. Nature, 509(7501), 439-446.
Xu, M., Pirtskhalava, T., Farr, J. N., Weigand, B. M., Palmer, A. K., Weivoda, M. M., … & Kirkland, J. L. (2018). Senolytics improve physical function and increase lifespan in old age. Nature Medicine, 24(8), 1246-1256.
Zhu, Y., Tchkonia, T., Fuhrmann-Stroissnigg, H., Dai, H. M., Ling, Y. Y., Stout, M. B., … & Kirkland, J. L. (2016). Identification of a novel senolytic agent, navitoclax, targeting the aged microenvironment. Aging Cell, 15(2), 428-435.
Recent Articles 
Integrative Perspectives on Cognition, Emotion, and Digital Behavior

Sleep-related:
Longevity/Nutrition & Diet:
Philosophical / Happiness:
Other:
Modern Mind Unveiled
Developed under the direction of David McAuley, Pharm.D., this collection explores what it means to think, feel, and connect in the modern world. Drawing upon decades of clinical experience and digital innovation, Dr. McAuley and the GlobalRPh initiative translate complex scientific ideas into clear, usable insights for clinicians, educators, and students.
The series investigates essential themes—cognitive bias, emotional regulation, digital attention, and meaning-making—revealing how the modern mind adapts to information overload, uncertainty, and constant stimulation.
At its core, the project reflects GlobalRPh’s commitment to advancing evidence-based medical education and clinical decision support. Yet it also moves beyond pharmacotherapy, examining the psychological and behavioral dimensions that shape how healthcare professionals think, learn, and lead.
Through a synthesis of empirical research and philosophical reflection, Modern Mind Unveiled deepens our understanding of both the strengths and vulnerabilities of the human mind. It invites readers to see medicine not merely as a science of intervention, but as a discipline of perception, empathy, and awareness—an approach essential for thoughtful practice in the 21st century.
The Six Core Themes
I. Human Behavior and Cognitive Patterns
Examining the often-unconscious mechanisms that guide human choice—how we navigate uncertainty, balance logic with intuition, and adapt through seemingly irrational behavior.
II. Emotion, Relationships, and Social Dynamics
Investigating the structure of empathy, the psychology of belonging, and the influence of abundance and selectivity on modern social connection.
III. Technology, Media, and the Digital Mind
Analyzing how digital environments reshape cognition, attention, and identity—exploring ideas such as gamification, information overload, and cognitive “nutrition” in online spaces.
IV. Cognitive Bias, Memory, and Decision Architecture
Exploring how memory, prediction, and self-awareness interact in decision-making, and how external systems increasingly serve as extensions of thought.
V. Habits, Health, and Psychological Resilience
Understanding how habits sustain or erode well-being—considering anhedonia, creative rest, and the restoration of mental balance in demanding professional and personal contexts.
VI. Philosophy, Meaning, and the Self
Reflecting on continuity of identity, the pursuit of coherence, and the construction of meaning amid existential and informational noise.
Keywords
Cognitive Science • Behavioral Psychology • Digital Media • Emotional Regulation • Attention • Decision-Making • Empathy • Memory • Bias • Mental Health • Technology and Identity • Human Behavior • Meaning-Making • Social Connection • Modern Mind
Video Section 

