Tachycardia in Septic Patients: When Should Beta Blockers Be Considered?

Abstract
Purpose: Tachycardia is a hallmark of sepsis, traditionally viewed as a compensatory mechanism to maintain cardiac output in the setting of reduced systemic vascular resistance. However, persistent tachycardia in septic patients has been associated with poor outcomes and may represent excessive sympathetic activation rather than beneficial compensation. This paper examines the current evidence regarding beta-blocker therapy in septic patients with persistent tachycardia.
Methods: A comprehensive literature review was conducted examining recent clinical trials, meta-analyses, and expert consensus statements on beta-blocker use in sepsis. The analysis focused on the pathophysiology of sepsis-induced tachycardia, clinical outcomes with beta-blocker therapy, safety considerations, and optimal patient selection criteria.
Results: Current evidence suggests that Long-term beta-blocker medication was linked to a reduced mortality rate in patients hospitalised with sepsis in internal medicine units who had absolute and relative tachycardia [1]. Meta-analyses of ultrashort-acting beta-blockers reveal conflicting findings. While more recent analyses indicate that the use of esmolol or landiolol did not lower mortality in patients with sepsis and persistent tachycardia, earlier studies showed a significant association with lower 28-day mortality (risk ratio, 0.68; 95% CI, 0.54-0.85; P <.001) [2] [3], while more recent analyses suggest the use of esmolol or landiolol did not reduce mortality in patients with sepsis with persistent tachycardia [4] [5].
Conclusions: Beta-blocker therapy in septic patients with persistent tachycardia represents a promising but evolving therapeutic strategy. Current evidence supports careful consideration of beta-blockers in select patients after initial resuscitation, with close hemodynamic monitoring. Further research with larger, well-designed multicenter trials is needed to establish definitive guidelines for optimal patient selection and timing of therapy.
Keywords: sepsis, tachycardia, beta-blockers, esmolol, landiolol, mortality, hemodynamic monitoring
Introduction
Sepsis continues to be a predominant cause of mortality among critically ill patients, as evidenced by global, regional, and national incidence and mortality rates from 1990 to 2017, analysed for the Global Burden of Disease Study [6] [7], demonstrating the continued significant burden of this condition worldwide. The pathophysiology of sepsis involves a complex interplay of inflammatory, hemodynamic, and metabolic derangements that contribute to the development of organ dysfunction and, ultimately, patient mortality.
Tachycardia is a cardinal sign of sepsis and septic shock, traditionally viewed through the lens of compensatory physiology. Although the cardiovascular system appears hyperdynamic, and tachycardia is mediated via the sympathetic nervous system and adrenal catecholamines, ventricular function is abnormal [8] [9]. However, emerging evidence challenges the assumption that all tachycardia in sepsis is beneficial, suggesting instead that persistent elevation of heart rate may contribute to adverse outcomes through multiple mechanisms.
The concept of using beta-blockers in septic shock has evolved significantly over the past two decades. Historically, β-blockers have been thought to be dangerous for septic shock because they can slow down the heart [10] [11]. However, there is a growing interest in using β-blockers to treat sepsis patients who still have tachycardia after initial resuscitation [12].
This analytical review aims to provide a comprehensive, neutral examination of the current evidence regarding beta-blocker therapy in septic patients with persistent tachycardia. We will explore the pathophysiological rationale, review clinical evidence from recent trials and meta-analyses, examine safety considerations, and discuss the practical implications for clinical decision-making. The analysis will maintain objectivity while critically evaluating both supporting and contradictory evidence to provide readers with a balanced perspective on this evolving therapeutic strategy.
Pathophysiology of Sepsis-Induced Tachycardia
Catecholamine Release and Sympathetic Activation
The pathophysiology of sepsis-induced tachycardia is multifactorial and complex. Septic shock is associated with massive release of endogenous catecholamines [13] [14]. This catecholamine surge serves as a central mechanism driving the cardiovascular manifestations of sepsis, including the characteristic tachycardia observed in these patients.
Among many factors causing sepsis-induced cardiac dysfunction, sympathetic nerve overstimulation, due to endogenous elevated catecholamine levels and exogenous catecholamine administration, is thought to play a significant role [15] [16]. The sustained elevation of catecholamines is well-documented in septic patients, with circulating levels of catecholamines being elevated in both early and late stages of polymicrobial sepsis [17].
Mechanisms of Catecholamine-Induced Cardiac Dysfunction
The excessive catecholamine exposure in sepsis contributes to cardiac dysfunction through several mechanisms:
- Direct Cardiotoxicity: Adrenergic agents may exacerbate catecholamine toxicity and contribute to poor outcomes [18] [19]. The cardiac effects include tachyarrhythmias, stress-related cardiomyopathy, cardiotoxicity with the characteristic finding of contraction band necrosis, digital and splanchnic ischemia, insulin resistance, immune suppression with enhanced bacterial growth, and increases in lipolysis, metabolic inefficiency, and catabolism [20].
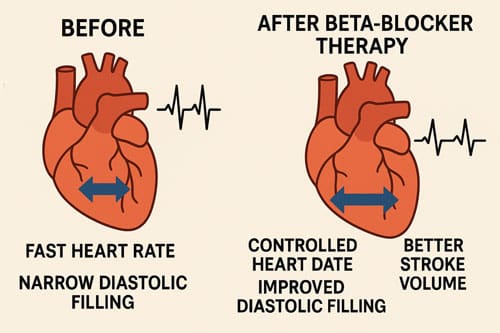
- Impaired Diastolic Function: Sustained tachycardia reduces diastolic filling time, potentially compromising stroke volume and coronary perfusion. Whether tachycardia is a direct contributor to mortality through impaired diastolic relaxation and reduced pumping efficiency is uncertain [21].
- Myocardial Oxygen Demand: Tachycardia increases myocardial oxygen consumption while potentially reducing coronary perfusion pressure during diastole, creating a supply-demand mismatch.
Inflammatory and Metabolic Consequences
Beyond direct cardiac effects, excessive sympathetic activation affects multiple organ systems:
Immune Modulation: Research conducted on healthy volunteers has demonstrated that catecholamines inhibit the lipopolysaccharide (LPS)-induced synthesis of tumour necrosis factor (TNF)alpha, interleukin (IL)-6, and IL-1beta. We expanded upon this observation and demonstrated that this effect is contingent upon alterations in the mRNA concentration of these cytokines [22]. However, the clinical significance of this immune modulation in septic patients remains complex and context-dependent.
Metabolic Effects: Catecholamines may impact the pathophysiology of sepsis by attenuating proinflammatory cytokine and augmenting antiinflammatory cytokine production by macrophages [23] [24]. This dual role highlights the complexity of catecholamine effects in sepsis.
Relative vs. Absolute Tachycardia
An essential concept in sepsis-related tachycardia is the distinction between an appropriate physiological response and excessive sympathetic activation. Our data are compatible with the hypothesis that some patients with sepsis experience an excess activation of the sympathetic nervous system, leading to a fatal outcome [25]. The concept of relative tachycardia, where heart rate is disproportionately elevated relative to temperature, has emerged as a potential marker of adverse outcomes. The pulse/temperature ratio was significantly higher in patients who died than in survivors: mean +/- SD 2.55 +/- 0.57 vs. 2.40 +/- 0.48 bpm/ degrees C (p < 0.0001). Excessive tachycardia was significantly associated with mortality in a logistic model accounting for other strong predictors of mortality (OR 1.54, 95%CI 1.10-2.17) [26].
Clinical Evidence for Beta-Blocker Therapy
Early Pioneering Studies
The clinical investigation of beta-blockers in sepsis began with small observational studies and has evolved to include larger randomized controlled trials. One of the most influential early studies was conducted by Morelli et al., which demonstrated promising results for esmolol therapy in septic shock patients.
The mortality rate after twenty-eight days was 49.4% in the esmolol group and 80.5% in the control group (adjusted hazard ratio, 0.39; 95% CI, 0.26 to 0.59; P < .001). [27] [28]. This study demonstrated that the targeted heart rates were attained in all patients within the esmolol group, in contrast to those in the control group. The median AUC for heart rate during the first 96 hours was -28/min (IQR, -37 to -21) for the esmolol group and -6/min (95% CI, -14 to 0) for the control group, with a mean reduction of 18/min (P < .001) [29].
The hemodynamic effects were encouraging, with β-blocker therapy associated with an increased stroke volume and left ventricular work index when compared to the control group. These favorable hemodynamic effects were associated with a better control of lactate levels, a higher reduction in norepinephrine, and fluid requirements [30].
Meta-Analyses and Systematic Reviews
Earlier Optimistic Meta-Analyses
Initial meta-analyses of beta-blocker therapy in sepsis showed promising results. The majority of the trials assessed in this review displayed beneficial results for beta-blocker use in patients with sepsis [31] [32]. A comprehensive meta-analysis by Hasegawa et al. found that the use of ultrashort-acting β-blockers such as esmolol and landiolol in patients with sepsis with persistent tachycardia despite initial resuscitation was associated with significantly lower 28-day mortality [33] [34].
Specifically, this meta-analysis demonstrated Esmolol or landiolol use in patients with sepsis and septic shock was significantly associated with lower 28-day mortality (risk ratio, 0.68; 95% CI, 0.54-0.85; P < .001) [35] [36], with The absolute risk reduction and number of patients to be treated to prevent one death were 18.2% and 5.5, respectively [37] [38].
Recent Contradictory Evidence
However, more recent and larger meta-analyses have challenged these earlier optimistic findings. A comprehensive 2024 meta-analysis by Sato et al. included eight RCTs (885 patients) in the primary analysis. Ultra-short-acting β-blockers did not improve mortality significantly at the most extended follow-up (risk ratio [RR], 0.84; 95% CI, 0.68-1.02; P = .08; I2 = 51%; very low certainty of the evidence) and 28-day mortality (RR, 0.77; 95% CI, 0.59-1.00; P = .05; I2 = 62%) [39].
Importantly, Subgroup analyses of mortality outcomes pointed toward different results between single-center and multicenter RCTs [40] [41]. This finding suggests that the benefits observed in smaller, single-center studies may not translate to larger, more heterogeneous patient populations.
Another recent meta-analysis reached similar conclusions, finding that Administration of short-acting betablockers did not reduce short-term mortality in septic patients with persistent tachycardia [42] and Short-term mortality as well as pooled mortality (most extended period of data on mortality) was not significantly impacted by treatment with short-acting betablockers when compared to the reference treatment (Risk difference, – 0.10 [95% CI, – 0.22 to 0.02]; p = 0.11; p for Cochran’s Q test = 0.001; I2 = 73%) [43].
Multicenter Randomized Controlled Trials
The STRESS-L Trial
The STRESS-L trial represents one of the most extensive multicenter randomized controlled trials examining landiolol in septic patients. An open-label, multicenter, randomized trial involving 126 adults (≥18 years) with tachycardia (heart rate ≥95/min) and established septic shock treated for at least 24 hours with continuous norepinephrine (≥0.1 μg/kg/min) in 40 UK National Health Service intensive care units. The trial ran from April 2018 to December 2021, with early termination in December 2021 due to a signal of possible harm [44].
The LANDI-SEP Trial
The LANDI-SEP trial, another extensive multicenter study, has provided additional evidence regarding landiolol use in septic shock patients. While specific results from this trial are still being analyzed, the study design represents the type of large-scale, multicenter investigation needed to provide definitive answers about beta-blocker efficacy in sepsis.
Real-World Evidence
Observational studies from large databases have provided additional insights. A study using the MIMIC-IV database found that There was no significant difference in 28-day mortality between two groups before (HR = 0.90, 95% CI = 0.73-1.12, p = 0.343) and after PSM (HR = 0.84, 95% CI = 0.6 Similar results were shown in 90-day mortality before (HR = 0.93, 95% CI = 0.75-1.14, p = 0.484) and after PSM (HR = 0.85, 95% CI = 0.67-1.09, p = 0.193) [45].
However, this study also revealed essential safety concerns: esmolol treatment was associated with a higher requirement of vasopressor use before (HR = 2.89, 95% CI = 2.18-3.82, p < 0.001) and after PSM (HR = 2.66, 95% CI = 2.06-3.45, p < 0.001) [46].
Hemodynamic Effects and Safety Considerations
Cardiovascular Effects
The hemodynamic effects of beta-blockers in septic patients are complex and sometimes counterintuitive. Clinical data suggest that the use of β-blockers for heart rate control in septic shock is safe when started at a low dose [47] [48]. However, while the available evidence suggests that the use of β-blockers in septic shock is safe, the effects on hemodynamics are controversial [49] [50].
Cardiac Output and Stroke Volume
Interestingly, despite reducing heart rate, beta-blockers may maintain or even improve cardiac output in some patients. Animal studies have demonstrated either a maintenance or an increase in cardiac output (CO) despite the decrease in heart rate (HR) associated with improved myocardial performance [51] [52]. This paradoxical effect may be explained by improved diastolic filling and enhanced myocardial efficiency.
Studies have shown that Cardiac selective beta-adrenergic blockade with esmolol reduces cardiac output in proportion to the percentage decreases in heart rate in moderately severe septic patients without adversely affecting oxygen utilization or hepatic or peripheral blood flow [53]. This proportional reduction suggests that stroke volume is preserved when heart rate is controlled.
Blood Pressure and Vasopressor Requirements
The effects on blood pressure and vasopressor requirements are variable. Some studies have shown benefits in terms of reduced vasopressor requirements, while others have demonstrated increased needs for hemodynamic support. For norepinephrine, -0.11 μg/kg/min (IQR, -0.46 to 0.02) for the esmolol group vs -0.01 μg/kg/min (IQR, -0.2 to 0.44) for the control group (P = .003) [54], suggesting potential vasopressor-sparing effects in some patients.
However, real-world data suggest caution, as esmolol treatment was associated with a higher requirement of vasopressor use [55] in an extensive database study.
Safety Profile and Contraindications
Traditional Contraindications
Historically, β-blockers have been considered to be relatively contraindicated for septic shock because they may cause cardiac suppression [56]. This traditional view has been challenged by recent evidence, but essential safety considerations remain.
Current Safety Recommendations
Recent expert consensus suggests specific criteria for safe beta-blocker use: Recent publications (2019–2021) on adrenergic β1 receptor antagonists used in septic shock indicate that esmolol and landiolol should not be used in the early phase. While there is no optimal timing for their administration, a minimum of 12 h after the initiation of vasopressor therapy in stabilized euvolemic patients is a reasonable option [57].
Additional safety criteria include: Patients should have a normal cardiac function, although a slight depression is compatible with landiolol use under hemodynamic monitoring. Slow titration in patients who remain tachycardic is preferable to rapid titration [58].
Monitoring Requirements
The use of beta-blockers in septic patients requires intensive hemodynamic monitoring. Hemodynamic monitoring plays a key role in the management of the critically ill and is used to identify hemodynamic instability and its cause and to monitor response to therapy. This article examines emerging technologies as well as more established techniques used to monitor hemodynamics in sepsis and assesses their potential roles in optimization of sepsis-induced tissue hypoperfusion [59] [60].
Comparative Efficacy: Esmolol vs. Landiolol
Recent comparative studies have examined differences between ultra-short-acting beta-blockers. Landiolol provides superior heart rate control in critically ill patients with tachycardia compared to Esmolol, without increasing vasopressor requirements during the first 24 h. Findings from ScvO2 levels and PCO2 gap suggest that Landiolol may exert less impact on cardiac output than Esmolol [61] [62].
This finding suggests that landiolol may offer some advantages over esmolol in terms of hemodynamic stability, potentially due to its more selective β1-receptor antagonism and shorter half-life.

Patient Selection and Timing
Identifying Appropriate Candidates
The selection of appropriate candidates for beta-blocker therapy in sepsis remains a critical clinical decision. Patients were stratified according to absolute tachycardia (HR ≥100/min) or relative tachycardia at presentation (tachycardia index above the third quartile, with tachycardia index defined as the ratio of HR to temperature) [63].
Current evidence suggests that patients most likely to benefit include:
- Persistent Tachycardia: Patients with heart rates persistently elevated despite adequate resuscitation
- Hemodynamic Stability: Patients who have achieved initial hemodynamic stabilization
- Adequate Cardiac Function: Patients without significant cardiac dysfunction
Timing of Initiation
The timing of beta-blocker initiation appears critical for safety and efficacy. Recent publications (2019-2021) on adrenergic β1 receptor antagonists used in septic shock indicate that esmolol and landiolol should not be used in the early phase. While there is no optimal timing for their administration, a minimum of 12 h after the initiation of vasopressor therapy in stabilized euvolemic patients is a reasonable option [64].
This recommendation reflects the understanding that early sepsis requires aggressive hemodynamic support, and beta-blocker therapy should only be considered once initial resuscitation goals have been achieved.
Exclusion Criteria
Several conditions represent contraindications to beta-blocker therapy in septic patients:
- Severe Hypotension: Patients with refractory hypotension despite maximal vasopressor support
- Significant Cardiac Dysfunction: Patients with severely reduced ejection fraction or cardiogenic shock
- Active Arrhythmias: Patients with significant bradycardia or high-grade heart block
- Early Resuscitation Phase: Patients in the initial hours of septic shock management
Monitoring Parameters
Patients receiving beta-blockers require intensive monitoring, including:
- Continuous Hemodynamic Monitoring: Blood pressure, heart rate, and rhythm
- Cardiac Output Assessment: Either through invasive or non-invasive methods
- Tissue Perfusion Markers: Lactate levels, urine output, mental status
- Vasopressor Requirements: Monitoring for increased needs
Contemporary Guidelines and Expert Consensus
Surviving Sepsis Campaign Guidelines
The Surviving Sepsis Campaign Guidelines represent the international standard for sepsis management. However, Several discrepancies exist between the randomized controlled trials with mortality differences in septic patients and the latest Surviving Sepsis Campaign Guidelines. This systematic review can be of help for improving future guidelines and may guide research on specific promising topics [65].
Notably, Five of the interventions with survival benefit in literature (vitamin C, terlipressin, polymyxin B, liberal transfusion strategy and immunoglobulins) were recommended to avoid in the Guidelines, while 44 interventions were not mentioned, including three interventions (esmolol, omega 3, and external warming) with at least two randomized controlled trials with a documented survival benefit [66].
This discrepancy highlights the challenge of translating research evidence into clinical practice guidelines, particularly for interventions like beta-blockers that require careful patient selection and monitoring.
Expert Society Recommendations
Various critical care societies have begun to address beta-blocker use in sepsis. The role of beta-blocker drugs in critically ill patients: a SIAARTI expert consensus statement [67] guides the appropriate use of these agents in the intensive care setting.
Clinical Practice Patterns
Real-world practice regarding beta-blockers in sepsis varies significantly. Chronic beta-blocker medication was discontinued during the acute phase of sepsis in 129 patients and continued in 167 patients. Continuation of beta-blocker therapy was significantly associated with decreased hospital (P=0.03), 28-day (P=0.04), and 90-day mortality rates (40.7% vs 52.7%; P=0.046) [68].
This finding regarding continuation of pre-existing beta-blocker therapy suggests that abrupt discontinuation may be harmful, providing additional support for the potential benefits of beta-blockade in septic patients.

Mechanisms of Benefit and Potential Harm
Theoretical Benefits
Cardiovascular Protection
Β-adrenergic blockade therapy may likely result in further beneficial effects in patients with sepsis, such as the reduction of inflammatory cytokine production, suppression of hypermetabolic status, maintenance of glucose homeostasis, and improvement of coagulation disorders [69] [70].
The cardiovascular protective effects may include:
- Reduced Myocardial Oxygen Consumption: Lower heart rate reduces myocardial oxygen demand
- Improved Diastolic Filling: Longer diastolic periods allow better ventricular filling
- Enhanced Coronary Perfusion: Improved diastolic perfusion pressure
- Reduced Catecholamine Toxicity: Protection against excessive adrenergic stimulation
Anti-Inflammatory Effects
Activating Beta-2 receptors and blocking Beta-1 receptors reduces the proinflammatory response by influencing cytokine production [71]. This immunomodulatory effect may contribute to improved outcomes beyond the direct cardiovascular benefits.
Metabolic Benefits
The physiological rationale behind the clinical application of β-blockers in septic shock is not limited to the modulation of the cardiovascular effects, but also to coagulopathy, attenuation of the hypermetabolic state, and immune-modulating effects [72].
Potential Mechanisms of Harm
Reduced Compensatory Reserve
The primary concern with beta-blocker therapy in sepsis is the potential to impair compensatory mechanisms. Despite this theoretical benefit of controlling the adverse effects of sepsis by β-antagonists, it is unclear if this mechanism truly benefits the cardiovascular system [73].
Masking of Clinical Deterioration
Beta-blockers may mask tachycardia as an early sign of clinical deterioration, potentially delaying recognition of worsening sepsis or new complications.
Hemodynamic Compromise
In patients with limited cardiac reserve, beta-blockade may precipitate hemodynamic collapse, particularly in the setting of ongoing sepsis and high catecholamine requirements.
Future Directions and Research Needs
Ongoing Clinical Trials
Several ongoing clinical trials are investigating beta-blocker therapy in sepsis. The design and results of these studies will likely influence future clinical practice and guideline recommendations.
Biomarkers and Patient Selection
Future research should focus on identifying biomarkers that can better predict which patients will benefit from beta-blocker therapy. Future studies should also provide extensive hemodynamic data to enable characterization of cardiac function before and during treatment [74] [75].
Precision Medicine Approaches
The diversity of sepsis suggests that a one-size-fits-all treatment approach may not be ideal. Consequently, forthcoming research ought to emphasise individualised strategies for beta-blocker therapy. It might include looking at genetic factors like beta-receptor polymorphisms and differences in how drugs are broken down, as well as using echocardiographic parameters and biomarkers to check heart function. Different sepsis phenotypes or endotypes will probably also react differently to beta-blockade, so separating patients into groups will be an essential part of future work.
Combination Therapies
Another critical area of research is the study of combination therapies. Examining the concurrent application of beta-blockers with other interventions may augment therapeutic benefits while concurrently mitigating potential risks.
Long-term Outcomes
Most recent evidence focuses on short-term mortality endpoints. However, subsequent studies ought to investigate the prolonged effects of beta-blocker therapy in individuals who have survived sepsis. This entails assessing the degree of cardiac function recovery, tracking functional outcomes and patient-reported quality of life, and examining healthcare utilisation via readmission rates and total expenses.
Clinical Decision-Making Framework
Assessment Algorithm
Based on current evidence, a systematic approach to beta-blocker consideration in septic patients might include:
Phase 1: Initial Assessment
A methodical evaluation of beta-blockers in septic patients may commence with a preliminary assessment. It would mean confirming the diagnosis of sepsis along with persistent tachycardia, which is usually defined as a heart rate of more than 95 to 100 beats per minute. Before starting, proper resuscitation must be done for at least 12 to 24 hours, and cardiac function and haemodynamic stability must be carefully checked.
Phase 2: Risk-Benefit Analysis
The next step would be to look at the pros and cons. It is essential to rule out contraindications like severe cardiac dysfunction or refractory hypotension and take into account factors that are unique to the patient, such as their age, comorbidities, and how bad their illness is. The choice also depends on whether there are enough resources to keep an eye on things.
Phase 3: Implementation
If beta-blocker therapy is undertaken, it should commence judiciously with minimal doses of ultra-short-acting agents. It is essential to monitor the patient’s blood flow closely, and there should be clear rules for when to stop treatment from the start.
Monitoring and Adjustment
Patients on beta-blockers need to be watched closely. It is essential to keep an eye on the ECG, blood pressure, and oxygen saturation at all times. It is also necessary to check the cardiac output and tissue perfusion often. Regular assessments should determine the efficacy of therapy and the appropriateness of its continuation.
Discontinuation Criteria
Treatment should be withdrawn if specific adverse developments occur. These encompass haemodynamic deterioration, exemplified by hypotension or increasing vasopressor requirements, novel or exacerbating cardiac dysfunction, or the onset of significant arrhythmias such as bradycardia or heart block. If there is no clinical improvement in tachycardia or the overall condition, treatment should also be stopped.
Limitations of Current Evidence
Study Heterogeneity
The results were not robust, and outcomes differed between single-center and multicenter RCTs [76] [77]. This heterogeneity makes it difficult to draw definitive conclusions about the efficacy of beta-blocker therapy across all septic populations.
Patient Selection Criteria
Variations in inclusion criteria among studies further constrain the generalisability of findings. For example, the severity of sepsis has been defined as ranging from sepsis to septic shock, and the thresholds for tachycardia have been established at different heart rate cutoffs. Likewise, the timing of intervention has varied, with specific trials commencing therapy early in the disease trajectory and others significantly later. These differences make it hard to compare studies directly.
Outcome Measures
While mortality is the most important outcome, studies have used different timepoints (28-day, hospital, ICU mortality), making meta-analysis challenging.
Monitoring Intensity
The level of hemodynamic monitoring has varied significantly across studies, potentially influencing both safety and efficacy outcomes.
Sample Size and Power
Many studies have been underpowered to detect clinically meaningful differences in mortality, and Trial sequence analyses showed that both mortality outcomes were not robust [78] [79].
Economic Considerations
Cost-Effectiveness Analysis
The economic consequences of beta-blocker therapy in sepsis are complex. Ultra-short-acting beta-blockers are very expensive, and the need for close monitoring adds to the cost of using them. On the other hand, there is a chance to save money by shortening the time patients spend in the ICU and the problems that come with it.
Resource Allocation
The intensive monitoring required for safe beta-blocker therapy may limit its applicability in resource-constrained settings. Future research should examine cost-effectiveness and optimal resource allocation strategies.
Ethical Considerations
Informed Consent
Due to the mixed evidence base and the potential for harm, informed consent for beta-blocker therapy in sepsis necessitates thorough discussion with patients or their surrogates. Clinicians must acknowledge the uncertainty surrounding potential benefits, be transparent about risks such as hemodynamic compromise and the need for close monitoring, and outline alternative approaches to managing tachycardia.
Research Ethics
Future trials must carefully balance the potential for benefit against the risk of harm, particularly given the mixed results from recent extensive multicenter studies.

Conclusion 
The use of beta-blockers in septic patients with persistent tachycardia represents a complex clinical decision that requires careful consideration of multiple factors. Current evidence presents a mixed picture: while early single-center studies and initial meta-analyses suggested significant mortality benefits, more recent large multicenter trials and updated meta-analyses have failed to confirm these benefits consistently.
The majority of the trials assessed in this review displayed beneficial results for beta-blocker use in patients with sepsis. However, owing to the deficit of large-scale randomized controlled trials addressing this topic, further research is needed to ensure the veracity of these results [80].
The pathophysiological rationale for beta-blocker therapy remains compelling. Tachycardia is also associated with poor outcomes in septic shock. When heart rate control was achieved with beta-adrenergic blockade, there was a signal towards improved outcomes [81] [82]. However, the translation of this theoretical benefit to consistent clinical outcomes has proven challenging.
Key conclusions from the current evidence include:
- Patient Selection is Critical: Not all septic patients with tachycardia are appropriate candidates for beta-blocker therapy. Selection criteria should include hemodynamic stability, adequate initial resuscitation, and absence of severe cardiac dysfunction.
- Timing Matters: Recent publications (2019-2021) on adrenergic β1 receptor antagonists used in septic shock indicate that esmolol and landiolol should not be used in the early phase [83] [84]. Beta-blockers should only be considered after initial resuscitation and stabilization.
- Intensive Monitoring is Essential: The safe use of beta-blockers in sepsis requires continuous hemodynamic monitoring and immediate availability of interventions to address potential complications.
- Evidence Quality Remains Limited: very low certainty of the evidence [85] characterizes much of the current literature, highlighting the need for larger, well-designed multicenter trials.
- Clinical Practice Should Remain Cautious: Given the mixed evidence and potential for harm, beta-blocker therapy should be considered experimental outside of carefully monitored research settings.
The field would benefit significantly from:
- Large, adequately powered multicenter randomized controlled trials with standardized protocols
- Development of validated biomarkers for patient selection
- Comprehensive economic analyses
- Long-term outcome studies
- Investigation of optimal dosing and titration strategies
Until such evidence becomes available, clinicians should approach beta-blocker therapy in septic patients with persistent tachycardia with appropriate caution, ensuring that robust monitoring capabilities are in place and that the decision is made as part of a comprehensive care plan tailored to individual patient circumstances.
The evolution of sepsis management continues to demonstrate the complexity of translating promising physiological concepts into improved patient outcomes. Beta-blocker therapy represents one such example where the theoretical rationale is compelling, but clinical implementation requires careful consideration of the available evidence, patient selection criteria, and safety monitoring requirements.
Key References
Alhazzani, W., Evans, L., Alshamsi, F., Møller, M. H., Ostermann, M., Prescott, H. C., … & Levy, M. M. (2021). Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Medicine, 47(11), 1181-1247.
Dellinger, R. P., Levy, M. M., Rhodes, A., Annane, D., Gerlach, H., Opal, S. M., … & Moreno, R. (2013). Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Critical Care Medicine, 41(2), 580-637.
Gore, D. C., & Wolfe, R. R. (2006). Hemodynamic and metabolic effects of selective beta1 adrenergic blockade during sepsis. Surgery, 139(5), 686-694.
Hasegawa, D., Sato, R., Prasitlumkum, N., Nishida, K., Takahashi, K., Yatabe, T., & Nishida, O. (2021). Effect of Ultrashort-Acting β-Blockers on Mortality in Patients With Sepsis With Persistent Tachycardia Despite Initial Resuscitation: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Chest, 159(6), 2289-2300.
Lee, Y. R., Seth, M. S., Soney, D., & Dai, H. (2019). Benefits of Beta-Blockade in Sepsis and Septic Shock: A Systematic Review. Clinical Drug Investigation, 39(5), 429-440.
Lescroart, M., Pequignot, B., Kimmoun, A., Klein, T., & Levy, B. (2022). Beta-blockers in septic shock: What is new?. Journal of Intensive Medicine, 2(3), 150-155.
Morelli, A., Ertmer, C., Westphal, M., Rehberg, S., Kampmeier, T., Ligges, S., … & Singer, M. (2013). Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA, 310(16), 1683-1691.
Rehberg, S., Joannidis, M., Morelli, A., & Duska, F. (2024). Landiolol for heart rate control in patients with septic shock and persistent tachycardia. A multicenter randomized clinical trial (Landi-SEP). Intensive Care Medicine, 50(10), 1622-1634.
Rudiger, A., & Singer, M. (2013). The heart in sepsis: from basic mechanisms to clinical management. Current Vascular Pharmacology, 11(2), 187-195.
Sato, R., Messina, S., Hasegawa, D., Santonocito, C., Scimonello, G., Sanfilippo, G., … & Sanfilippo, F. (2024). Mortality in Patients With Sepsis Treated With Esmolol or Landiolol: A Systematic Review and Meta-Analysis of Randomized Controlled Trials With Trial Sequential Analysis. Chest, 167(1), 121-138.
Singer, M., Deutschman, C. S., Seymour, C. W., Shankar-Hari, M., Annane, D., Bauer, M., … & Angus, D. C. (2016). The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA, 315(8), 801-810.
van Loon, L. M., van der Hoeven, J. G., Veltink, P. H., & Lemson, J. (2018). Hemodynamic response to β-blockers in severe sepsis and septic shock: A review of current literature. Journal of Critical Care, 50, 138-143.

