Neurocysticercosis: The Hidden Brain Parasite Crossing Global Borders in 2025
Introduction
Neurocysticercosis remains the most prevalent parasitic infection of the human central nervous system (CNS) and continues to represent a major global health challenge. Current estimates suggest that more than 50 million individuals are affected worldwide, with approximately 50,000 deaths attributed to the condition each year. The disease results from infection with the larval stage of Taenia solium, the pork tapeworm, which gains access to the CNS following ingestion of parasite eggs. Once in the brain or spinal cord, the larvae form cystic lesions that can trigger a wide spectrum of neurological complications. These range from seizures and chronic epilepsy to hydrocephalus, focal neurological deficits, and, in severe cases, death.
Epidemiologically, neurocysticercosis is one of the leading causes of acquired epilepsy in endemic regions. Latin America, sub-Saharan Africa, and vast areas of Asia, including the Indian subcontinent, Southeast Asia, and China, have long been recognized as hotspots of transmission. In these regions, the persistence of traditional pig-rearing practices, limited access to clean water, and inadequate sanitation contribute significantly to the ongoing cycle of infection. The World Health Organization has classified T. solium as a neglected tropical disease and has highlighted its burden on public health. In 2015 alone, the parasite was estimated to account for 2.8 million disability-adjusted life-years (DALYs), underscoring both the morbidity and mortality associated with infection.
Importantly, neurocysticercosis is no longer confined to developing nations. Over the past several decades, increased global migration and international travel have shifted the epidemiological landscape. Industrialized countries that were previously considered non-endemic are now reporting higher case numbers, particularly within immigrant populations originating from endemic regions. This evolving pattern of distribution requires neurologists, infectious disease specialists, and other healthcare professionals worldwide to develop a working knowledge of the condition’s clinical features and management strategies.
The clinical presentation of neurocysticercosis is notably variable and depends on the number, location, and viability of the cysts within the CNS, as well as the host’s immune response. Seizures remain the most common manifestation, but other presentations include chronic headaches, cognitive impairment, psychiatric symptoms, and intracranial hypertension due to obstructive hydrocephalus. Diagnosis is often challenging and typically requires a combination of neuroimaging, serological testing, and clinical correlation. Magnetic resonance imaging (MRI) and computed tomography (CT) remain central to detecting cystic lesions and differentiating active from calcified disease.
Management of neurocysticercosis involves a multifaceted approach. Antiparasitic therapy with albendazole or praziquantel, often in combination with corticosteroids to reduce inflammatory responses, is the cornerstone of treatment. In cases of raised intracranial pressure or hydrocephalus, neurosurgical intervention such as ventriculoperitoneal shunting may be required. Long-term antiepileptic therapy is frequently necessary to control seizures, and individualized treatment decisions should be guided by cyst location, stage, and symptomatology.
Despite advances in diagnosis and treatment, significant gaps remain in global awareness, preventive strategies, and healthcare infrastructure. Control of T. solium transmission relies heavily on improving sanitation, enforcing meat inspection standards, ensuring access to safe water, and implementing large-scale public health education. Vaccination and antiparasitic treatment of pigs, as well as targeted deworming programs in endemic populations, represent additional strategies that can disrupt the life cycle of the parasite.
In 2025, neurocysticercosis continues to exemplify the intersection of infectious disease, neurology, and global health inequities. As the parasite crosses traditional geographic boundaries, the condition increasingly challenges healthcare systems in both endemic and non-endemic regions. Familiarity with its complex lifecycle, diverse clinical presentations, and evidence-based management is now essential for clinicians worldwide. Addressing this neglected yet devastating infection requires sustained international collaboration, integration of clinical expertise, and investment in public health interventions that target both the human and animal reservoirs of T. solium.
Life Cycle and Transmission of Taenia solium in Humans and Pigs
The complex life cycle of Taenia solium involves two hosts and distinct disease manifestations depending on the stage of parasite development. Understanding these transmission patterns remains essential for developing effective prevention strategies against this neurologically devastating parasite.
Fecal-Oral Transmission and Autoinfection Pathways
Taenia solium transmission occurs primarily through the fecal-oral route. Humans acquire cysticercosis after ingesting eggs shed in the feces of a human tapeworm carrier. These microscopic eggs, immediately infectious without requiring a developmental period outside the host, contaminate food and water sources. Subsequently, oncospheres (embryos) hatch in the intestine, invade the intestinal wall, enter the bloodstream, and migrate to various tissues. In neurocysticercosis cases, these larvae specifically travel to the central nervous system.
Notably, autoinfection presents another transmission mechanism. This occurs when individuals who already harbor adult T. solium ingest the parasite’s eggs through poor hand hygiene after bowel movements. Furthermore, autoinfection may happen through reverse peristalsis, when proglottids (tapeworm segments) pass retrograde from the intestine to the stomach. This internal pathway allows the parasite to complete its life cycle within a single human host without requiring an intermediate host.
Which Worm Causes Neurocysticercosis: Role of Taenia solium
Neurocysticercosis develops specifically from Taenia solium, commonly known as the pork tapeworm. This parasitic cestode uniquely infects humans in two distinct ways—either as intestinal taeniasis (adult worm infection) or as tissue cysticercosis (larval stage infection). The adult tapeworm, measuring between 2-7 meters in length with approximately 1,000 proglottids, resides in the human small intestine.
Once cysticerci reach the central nervous system, they can remain viable with minimal inflammatory changes for extended periods, protected by the blood-brain barrier. As cysticerci mature, they progress through three stages: first becoming inflamed (colloidal stage), then breaking down (granular stage), and finally hardening into calcified nodules. Throughout these stages, diverse neurological complications manifest depending on the location and number of cysts.
Pig as Intermediate Host: Environmental Contamination Cycle
Pigs function as the primary intermediate host in the T. solium lifecycle. The cycle begins when pigs ingest T. solium eggs from human feces, often through contaminated vegetation or direct contact with fecally contaminated soil. After ingestion, the eggs evolve into oncospheres that penetrate the intestinal wall using their hooks and penetration gland enzymes.
These oncospheres then migrate through the bloodstream to various organs, with many forming cysticerci in striated muscles. A single cysticercus appears as a fluid-filled, spherical structure measuring 1-2 cm in diameter, containing an invaginated protoscolex. These cysts typically form within 70 days and may continue developing for up to a year.
The cycle completes when humans consume undercooked pork containing viable cysticerci. In the human intestine, the protoscolex evaginates, attaches to the intestinal mucosa using its crowned hooks and four suckers, and develops into an adult tapeworm over 10-12 weeks. This adult worm can survive for 2-5 years, continuously releasing proglottids containing up to 50,000 embryonated eggs each.
This parasitic life cycle perpetuates particularly in regions with poor sanitation, free-roaming pigs, and consumption of undercooked pork—creating conditions where humans and pigs maintain close ecological contact through environmental contamination.
Clinical Manifestations and Neurological Complications
Clinical presentations of neurocysticercosis vary dramatically based on the parasites’ location, number, size, and stage of development within the central nervous system. The host’s inflammatory response to these larval cysts creates a spectrum of neurological disorders ranging from completely asymptomatic cases to life-threatening complications.
Seizures and Epilepsy in Intraparenchymal Neurocysticercosis
Epilepsy emerges as the predominant clinical manifestation of parenchymal neurocysticercosis, occurring in 70-90% of symptomatic patients according to published case series. Parenchymal cysticercosis typically presents with seizures that respond well to antiepileptic medications, although cysts may survive for years with intermittent neurological symptoms. In regions where neurocysticercosis is endemic, approximately 30% of patients with epilepsy also present evidence of this parasitic infection.
The relationship between seizures and cyst stages is particularly noteworthy. Seizures occur most frequently during the degenerating (colloidal) phase when inflammation around dying cysts triggers epileptogenic activity. However, calcified lesions also maintain seizure potential, with recurrence rates at 6-12 months ranging from less than 10% to 34% in patients with only calcified lesions. This chronic epileptogenesis likely results from both local inflammation and the formation of reactive gliotic scars.
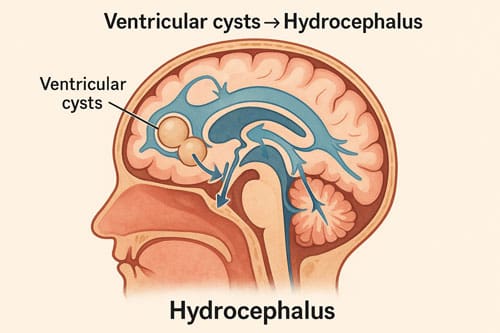
Hydrocephalus and Intracranial Hypertension in Ventricular Cysts
Hydrocephalus develops in nearly 30% of neurocysticercosis patients, creating potentially fatal intracranial pressure complications. Multiple pathogenic mechanisms contribute to hydrocephalus, including mechanical blockage of ventricles, obstruction of cerebrospinal fluid pathways, and communicating hydrocephalus from arachnoiditis.
Intraventricular cysts most commonly occur in the fourth ventricle (53%), followed by the third ventricle (27%), lateral ventricles (11%), and aqueduct (9%). These obstructions produce headaches, vomiting, and visual disturbances. Moreover, when cysts obstruct the fourth ventricle, patients may experience Bruns’ syndrome—sudden loss of consciousness related to head movements. Without neurosurgical intervention, ventricular neurocysticercosis carries high mortality rates.
Racemose Neurocysticercosis and Subarachnoid Involvement
Racemose neurocysticercosis represents a particularly aggressive variant, occurring in approximately 3.6% of neurocysticercosis patients. This form manifests as clusters of grape-like vesicles primarily in the subarachnoid spaces and basal cisterns, often without an identifiable scolex. Unlike parenchymal disease, racemose neurocysticercosis carries a poor prognosis with mortality rates ranging from 20-50% if not optimally treated.
Clinical manifestations of subarachnoid disease include severe headaches, intracranial hypertension, and cerebrovascular complications. Additionally, basilar arachnoiditis may cause communicating hydrocephalus or vasculitis, occasionally resulting in lacunar infarctions or large-vessel strokes. Inflammatory occlusion secondary to fibrous arachnoiditis represents the most common finding in these cases.
Neurocysticercosis Complication is Seen in Worm-Induced Inflammation
Inflammation fundamentally drives the pathophysiology of neurocysticercosis complications. When cysts degenerate, either naturally or after antiparasitic treatment, they release antigens that trigger robust inflammatory responses. This process involves increased expression of pro-inflammatory mediators including TNF-α, IL-6, and IFN-γ, alongside decreased levels of anti-inflammatory IL-10.
Cysticercotic encephalitis, a severe inflammatory form affecting primarily children and young women in their first three decades, presents with clouded consciousness, seizures, and intracranial hypertension. This condition results from hundreds of small viable or degenerating cysts producing diffuse cerebral edema.
Inflammation around degenerating cysts in the brain parenchyma typically triggers seizures, while inflammation in subarachnoid spaces causes diffuse or focal arachnoiditis. Consequently, antiparasitic drugs must be administered cautiously since larval death provokes inflammatory responses that may worsen symptoms—necessitating concurrent anti-inflammatory therapy.
Diagnostic Imaging and Serological Confirmation
Accurate diagnosis of neurocysticercosis requires a combination of neuroimaging and laboratory confirmation methods. The diagnostic approach varies based on cyst location, evolutionary stage, and the host’s immune response to the Taenia solium larvae.
MRI vs CT for Detecting Scolex and Cyst Stages
Neuroimaging stands as the cornerstone for neurocysticercosis diagnosis, with each modality offering distinct advantages. Computed tomography (CT) excels at identifying calcifications in the brain parenchyma, whereas magnetic resonance imaging (MRI) demonstrates superior sensitivity for detecting cysts in the ventricular system. The visualization of a scolex—appearing as a bright nodule within the cyst—creates the characteristic “hole-with-dot” imaging pattern that is considered pathognomonic for neurocysticercosis. In fact, this remains the only neuroimaging finding that should be considered definitively diagnostic.
Both imaging modalities reveal distinct features corresponding to the parasite’s evolutionary stages. In the vesicular stage, cysts appear with CSF-like density on CT and intensity on MRI, often revealing the eccentric scolex. As cysts progress to the colloidal vesicular stage, the fluid becomes hyperattenuating on CT and hyperintense on T1-weighted and FLAIR sequences, accompanied by surrounding edema and enhancing walls. Eventually, the granular nodular stage shows decreasing edema with the cyst retracting into a small enhancing nodule, before developing into the final calcified nodule stage with characteristic signal drop-out on T2 and T2* sequences.
EITB and Antigen-Detection ELISA Sensitivity
Enzyme-linked immunoelectrotransfer blot (EITB) assay represents the serologic test of choice for detecting antibodies against T. solium glycoprotein antigens. This method demonstrates a documented specificity approaching 100% and sensitivity between 94-98% for patients with multiple cystic lesions. Nevertheless, EITB exhibits a significant limitation—its sensitivity drops below 50% in patients with single intracranial cysticerci, creating diagnostic challenges for isolated lesions.
In contrast, antigen-detection ELISA offers higher specificity for identifying active viable infections. This assay proves especially valuable for diagnosing living cysticerci in the subarachnoid space at the base of the skull, with sensitivity rates of 87% and specificity of 95% when performed on cerebrospinal fluid (CSF). Thus, combining both antibody and antigen detection methods provides complementary information about the parasite’s viability status.
PCR in CSF for Confirmatory Diagnosis
Polymerase chain reaction (PCR) testing of cerebrospinal fluid has emerged as a powerful confirmatory tool, especially for extraparenchymal neurocysticercosis. PCR techniques targeting highly repetitive elements of the T. solium genome can detect as little as 10 femtograms of parasite DNA. Importantly, PCR demonstrates higher sensitivity than other diagnostic tests across all patient groups.
PCR holds exceptional value for subarachnoid and intraventricular neurocysticercosis, with sensitivity reaching 90.9% for extraparenchymal cases versus only 42.9% for exclusively parenchymal disease. Overall, PCR achieves 72.2% sensitivity with 100% specificity, outperforming other diagnostic methods. Real-time quantitative PCR detecting the repetitive Tsol13 sequence shows particular promise as both a diagnostic marker and a measure of cure, with 100% of CSF samples from active neurocysticercosis cases testing positive.
Treatment Protocols and Drug Regimens
Effective management of the Taenia solium larval infection requires tailored pharmacological and surgical approaches based on cyst location, number, and evolutionary stage. Treatment decisions must balance parasiticidal efficacy against the risk of exacerbating inflammation.
Albendazole vs Praziquantel: Dosage and Efficacy
Current evidence favors albendazole (15 mg/kg/day divided in two doses) as the first-line antiparasitic agent, administered for 10-14 days in standard regimens. For patients with more than two viable parenchymal cysticerci, combined therapy with albendazole and praziquantel (50 mg/kg/day) demonstrates superior efficacy—producing a threefold increase in complete cyst resolution compared to albendazole monotherapy. Chinese clinical trials involving 864 patients documented 96-98% effectiveness with combination regimens versus 55% with albendazole alone and 68% with praziquantel monotherapy.
Both medications exhibit different advantages. Albendazole penetrates cerebrospinal fluid more effectively and maintains therapeutic levels when administered with corticosteroids. Conversely, praziquantel levels may decrease with concurrent steroid use. For subarachnoid or racemose forms, higher albendazole dosing at 30 mg/kg/day often becomes necessary.
Corticosteroid Use to Control Inflammatory Reactions
Adjunctive corticosteroid therapy must begin before antiparasitic drugs in all treated patients. This approach mitigates the inflammatory cascade triggered by parasite death that otherwise causes edema and potential neurological deterioration. In patients with diffuse cerebral edema, corticosteroids serve as primary therapy while antiparasitic treatment should be delayed.
Importantly, the inflammatory response poses particular danger early in treatment. Among 450 patients receiving praziquantel, 87% experienced adverse reactions, including seizures (9%), increased intracranial pressure (18%), and mental symptoms (3%). Consequently, some practitioners recommend sequential rather than concurrent administration of antiparasitic medications, starting with albendazole followed by praziquantel.
Surgical Intervention for Ventricular and Racemose Cysts
For fourth ventricular cysticerci, surgical removal is preferred over medical therapy or shunt surgery. Minimally invasive neuroendoscopy represents the optimal approach for lateral and third ventricular cysts. Preoperative antiparasitic medications should be avoided since they may compromise successful cyst removal by disrupting parasite integrity.
Hydrocephalus frequently necessitates ventriculoperitoneal shunting, yet shunts often require multiple revisions due to obstruction. For racemose neurocysticercosis, surgical extirpation may be indicated despite risks of intraoperative cyst rupture causing potentially fatal inflammatory responses.
Niclosamide for Intestinal Taeniasis Without CNS Risk
Niclosamide emerges as the safest option for treating intestinal taeniasis without risking neurocysticercosis complications. Unlike systemically absorbed medications, niclosamide remains primarily in the intestinal tract. Adults receive 2g while children aged 2-6 years receive 1g. This agent shows 84.3% effectiveness against intestinal taeniasis with minimal adverse effects—primarily mild gastrointestinal disturbances.
Conversely, both albendazole and praziquantel must be used cautiously in patients with suspected cysticercosis due to reports of seizures potentially associated with treatment. This makes niclosamide particularly valuable in endemic regions where undiagnosed neurocysticercosis may coexist with intestinal taeniasis.

Global Spread and Public Health Implications in 2025
Taenia solium neurocysticercosis has transcended traditional geographic boundaries, creating new public health challenges worldwide. The international dissemination of this parasitic infection primarily stems from human migration patterns and travel between endemic and non-endemic regions.
Migration-Driven Cases in Non-Endemic Countries
The increasing prevalence of neurocysticercosis in developed nations has been largely attributed to immigration from endemic areas. In the United States, estimates suggest between 1,320 and 5,050 new cases occur annually. Most compelling evidence from Qatar indicates that over 98% of neurocysticercosis cases were identified in immigrants from endemic countries, with merely 1.2% occurring in local Qataris. Concurrently, in the United States, while most cases affect Hispanic immigrants, documented instances of local transmission exist, particularly when tapeworm carriers are present within households. Seroprevalence rates ranging from 18.3% to 25.8% have been detected among certain Asian and African refugee populations resettling in America.
Surveillance Strategies in Refugee and Immigrant Populations
Active surveillance remains essential for accurately determining neurocysticercosis incidence in non-endemic regions. Indeed, improved detection through neuroimaging has contributed substantially to case identification in high-income countries. Currently, surveillance efforts increasingly focus on specific high-risk groups, including domestic workers from Latin America and Southeast Asian immigrants. For effective monitoring, experts recommend implementing screening protocols at neuro-care centers for patients presenting with epilepsy, potentially revealing previously unrecognized endemic regions.
Eradication Programs: Porcine Vaccination and Mass Chemotherapy
The World Health Organization has established ambitious targets for intensified T. solium control, aiming to increase the number of countries with enhanced control measures from 2 in 2020 to 17 by 2030. Similarly, effective intervention strategies combine human and porcine treatments. The TSOL18 vaccine provides 99-100% protection against porcine cysticercosis, while mass chemotherapy with niclosamide or praziquantel targets human taeniasis. A comprehensive approach in Peru demonstrated the feasibility of interrupting transmission through integrated interventions including mass human chemotherapy with niclosamide, porcine treatment with oxfendazole, and pig vaccination. Furthermore, community-led sanitation programs addressing open defecation have proven valuable supplements to pharmaceutical interventions.

Conclusion 
Neurocysticercosis stands as a formidable parasitic challenge in 2025, transcending traditional geographic boundaries and demanding heightened clinical awareness among neurologists worldwide. This Taenia solium infection, once primarily confined to developing regions, now appears with alarming frequency in industrialized nations due to changing migration patterns. Consequently, healthcare practitioners across diverse settings must familiarize themselves with its complex presentations and management strategies.
The intricate lifecycle involving human-pig transmission underscores why prevention efforts require multifaceted approaches. Specifically, interventions targeting both human taeniasis and porcine cysticercosis show the greatest promise for disease control. Nonetheless, clinical management remains challenging given the varied manifestations—from seizures and epilepsy in parenchymal disease to potentially fatal hydrocephalus in ventricular involvement.
Diagnostic accuracy has improved substantially through combined neuroimaging and serological techniques. The pathognomonic “hole-with-dot” appearance on imaging provides definitive evidence, while newer PCR methodologies offer enhanced sensitivity for extraparenchymal cases. Therefore, clinicians must select diagnostic approaches tailored to suspected cyst location and evolutionary stage.
Treatment decisions likewise require careful individualization. Albendazole remains the first-line antiparasitic agent, though combination therapy with praziquantel demonstrates superior efficacy for multiple viable cysts. Additionally, corticosteroid administration before antiparasitic treatment proves essential to mitigate potentially dangerous inflammatory reactions. Surgical intervention, particularly neuroendoscopy, offers the best approach for ventricular and racemose cysts.
Public health strategies have evolved toward integrated control measures. These include human mass chemotherapy, porcine vaccination programs, and community-led sanitation improvements—approaches that together show promise for transmission interruption. The World Health Organization’s targets for enhanced control measures across more countries by 2030 reflect growing international commitment to addressing this parasite.
Though previously neglected, neurocysticercosis now demands attention as a truly global health concern. Healthcare systems in non-endemic regions must develop surveillance strategies focusing on high-risk populations, particularly immigrants and refugees from endemic areas. Furthermore, neurologists worldwide require thorough understanding of this condition’s manifestations, diagnosis, and management to provide optimal care as cases continue emerging beyond traditional boundaries. Undoubtedly, effective control of neurocysticercosis will require sustained collaboration between clinicians, public health officials, and affected communities across international borders.
Key Takeaways
Neurocysticercosis, caused by Taenia solium larvae in the brain, is rapidly spreading beyond endemic regions through global migration, requiring worldwide clinical awareness and updated diagnostic strategies.
- Taenia solium causes dual infections: intestinal tapeworms and brain cysts through fecal-oral transmission and autoinfection pathways • Seizures dominate symptoms: 70-90% of patients develop epilepsy, with highest risk during cyst degeneration phases • Combined imaging and serology improve diagnosis: MRI detects ventricular cysts while CT shows calcifications; PCR offers 100% specificity • Albendazole plus corticosteroids is first-line treatment: combination therapy with praziquantel triples cure rates for multiple cysts • Migration drives global spread: 98% of cases in non-endemic countries occur in immigrants from affected regions • Integrated control programs work: combining human chemotherapy, pig vaccination, and sanitation can interrupt transmission cycles
The “hole-with-dot” imaging pattern remains the only definitively diagnostic neuroimaging finding, while surgical intervention proves essential for ventricular cysts causing hydrocephalus.

Frequently Asked Questions:
FAQs
Q1. What is the prognosis for neurocysticercosis? The outlook for neurocysticercosis varies depending on the number and location of cysts. Cases with a single enhancing lesion generally have a favorable prognosis, with over 60% of lesions resolving within 6 months. However, more complex cases may require longer treatment and have a less predictable outcome.
Q2. Which parasite is responsible for causing neurocysticercosis? Neurocysticercosis is caused by the larval cysts of the pork tapeworm, Taenia solium. These cysts can infect various parts of the body, including the brain, leading to the condition known as neurocysticercosis.
Q3. How prevalent is neurocysticercosis in the United States? While precise incidence data for the US is limited, estimates suggest there are between 0.2 to 0.6 cases per 100,000 in the general population. Among Hispanic populations, the incidence is higher, ranging from 1.5 to 5.8 cases per 100,000.
Q4. What percentage of the global population is affected by brain parasites? While neurocysticercosis rates vary, another common brain parasite, Toxoplasma, is estimated to infect 30-50% of people worldwide. Most infections are asymptomatic, but the parasites can remain in the body for years as tiny tissue cysts.
Q5. How is neurocysticercosis diagnosed and treated? Diagnosis typically involves neuroimaging (MRI or CT scans) combined with serological tests. The characteristic “hole-with-dot” appearance on imaging is considered diagnostic. Treatment usually includes antiparasitic drugs like albendazole, often combined with corticosteroids to manage inflammation. In some cases, particularly for ventricular cysts, surgical intervention may be necessary.
References:
[1] – https://pmc.ncbi.nlm.nih.gov/articles/PMC3020422/
[2] – https://www.idsociety.org/practice-guideline/neurocysticercosis/
[3] – https://radiopaedia.org/articles/neurocysticercosis
[4] – https://pmc.ncbi.nlm.nih.gov/articles/PMC4005108/
[5] – https://pmc.ncbi.nlm.nih.gov/articles/PMC10156967/
[6] – https://pmc.ncbi.nlm.nih.gov/articles/PMC3349430/
[7] – https://www.thejcn.com/Synapse/Data/PDFData/0145JCN/jcn-10-363.pdf
[8] – https://www.cureus.com/articles/336154-racemose-neurocysticercosis-presenting-as-vasculitic-infarct-and-obstructive-hydrocephalus-a-rare-presentation
[9] – https://pmc.ncbi.nlm.nih.gov/articles/PMC8216685/
[10] – https://emedicine.medscape.com/article/215589-overview
[11] – https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0003577
[12] – https://www.cdc.gov/cysticercosis/hcp/diagnosis-testing/index.html
[13] – https://pmc.ncbi.nlm.nih.gov/articles/PMC2912527/
[14] – https://journals.asm.org/doi/10.1128/jcm.01550-21
[15] – https://pmc.ncbi.nlm.nih.gov/articles/PMC5241005/
[16] – https://www.frontiersin.org/journals/veterinary-science/articles/10.3389/fvets.2021.615703/full
[17] – https://www.neurology.org/doi/10.1212/WNL.0000000000212432
[18] – https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(15)70022-8/fulltext
[19] – https://pmc.ncbi.nlm.nih.gov/articles/PMC126865/
[20] – https://surgicalneurologyint.com/surgicalint-articles/treatment-of-racemose-neurocysticercosis/
[21] – https://www.thelancet.com/journals/lanam/article/PIIS2667-193X(24)00203-5/fulltext
[22] – https://www.who.int/news-room/fact-sheets/detail/taeniasis-cysticercosis
[23] – https://www.cdc.gov/taeniasis/hcp/clinical-care/index.html
[24] – https://researchexperts.utmb.edu/en/publications/neurocysticercosis-in-the-united-states
[25] – https://www.sciencedirect.com/science/article/pii/S1995764517305795
[26] – https://pmc.ncbi.nlm.nih.gov/articles/PMC8141574/
[27] – https://pmc.ncbi.nlm.nih.gov/articles/PMC6390339/
[28] – https://www.who.int/activities/supporting-countries-in-their-cysticercosis-control-efforts

