Cardiac Amyloidosis: The Missing Diagnosis in Preserved Heart Failure Cases

Introduction
Heart failure with preserved ejection fraction (HFpEF) affects about one-third of people with heart failure, making it a difficult condition for doctors to diagnose. Even though HFpEF is common, it often hides a serious underlying condition called cardiac amyloidosis. This infiltrative cardiomyopathy, characterized by the accumulation of extracellular proteins in heart tissue, can significantly impact prognosis yet remains frequently undiagnosed.
According to recent evidence, only 10% of medical practitioners perform systematic screening for cardiac amyloidosis in HFpEF patients, despite two-thirds reporting experience in diagnosing the condition. This diagnostic gap is particularly concerning because cardiac amyloidosis, sometimes called “stiff heart syndrome,” disrupts normal cardiac structure and function by replacing healthy heart muscle with amyloid deposits. Consequently, patients develop diastolic dysfunction that manifests as HFpEF. The clinical impact is substantial—suspected cardiac amyloidosis patients demonstrate poor functional status with a median New York Heart Association score of 3.00 and diminished quality of life scores of 42.00.
This article looks at the similarities between amyloid heart disease and HFpEF, points out important warning signs that should lead to more investigations, and explores how the symptom burden is related to functional deterioration. Additionally, it assesses screening methodologies that could assist practitioners in recognizing this “missing diagnosis” sooner in clinical practice, consequently enhancing results for this difficult patient demographic.
Symptom Overlap Between HFpEF and Cardiac Amyloidosis
Cardiac amyloidosis presents a diagnostic conundrum when embedded within the broader HFpEF syndrome. The clinical presentations of these conditions converge in ways that often lead to delayed recognition of amyloid heart disease. Understanding these overlapping manifestations proves crucial for timely diagnosis and appropriate management.
Fatigue, Dyspnea, and Exercise Intolerance in Both Conditions
The cardinal symptoms of cardiac amyloidosis mirror those typically seen in HFpEF. Patients with both conditions experience debilitating physical symptoms, including exercise intolerance, fatigue, and dyspnea, which collectively diminish functional capacity and quality of life. These shared manifestations often create a diagnostic blind spot for clinicians.
Dyspnea on exertion represents a hallmark symptom in both cardiac amyloidosis and HFpEF. Initially ascribed to the heart’s failure to satisfy metabolic demands during physical exertion, the fundamental mechanisms are, in fact, significantly more intricate. Patients with amyloid heart disease often experience shortness of breath during physical activity or while supine, accompanied by orthopnea and paroxysmal nocturnal dyspnea—symptoms that are indistinguishable from those observed in non-amyloid HFpEF.
Exercise intolerance and early fatigue are common to both conditions, but they arise from different pathophysiological changes in cardiac amyloidosis. Individuals with ATTR cardiac amyloidosis (ATTR-CM) exhibit a significant inability to augment stroke volume during exertion owing to restrictive left ventricular filling. Moreover, the sustained physical activity invariably leads to reduced capacity for generating maximal force or power—commonly defined as muscle fatigue.
Notable differences exist, nonetheless, in symptom constellation. Cardiac amyloidosis patients may additionally exhibit systemic manifestations absent in typical HFpEF, including polyneuropathy and gastrointestinal disorders. Unfortunately, these distinguishing features often emerge late in the disease course, after significant cardiac involvement has already occurred.
Why Amyloid Heart Disease Mimics Stiff Heart Failure
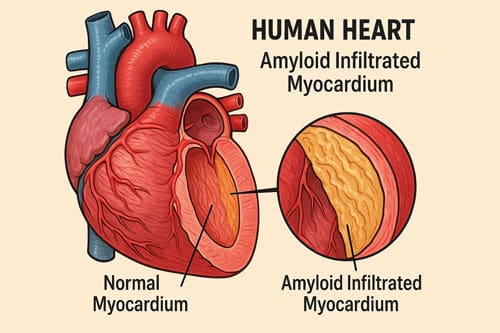
The term “stiff heart syndrome” aptly describes the physiological changes underlying both conditions. In cardiac amyloidosis, the deposition of amyloid proteins directly disturbs the normal structure of the myocardium. As these proteins build up in the heart muscle, the walls get thicker and the heart gets bigger, making it harder to keep the heart’s output at a healthy level. This process is similar to the stiffening of the wall that happens in HFpEF, but it happens in a completely different way.
The pathophysiology encompasses more than mere mechanical disruption. Soluble monomers and oligomers in AL and ATTR amyloidosis directly harm cardiomyocytes. These pre-fibrillar compounds raise the levels of reactive oxygen species, change how calcium moves in and out of cells, and start pathways that cause contractile dysfunction, problems with releasing cardiac muscle cells, and faster apoptosis.
The resulting cardiac impairment manifests as diastolic dysfunction—the heart cannot relax properly during the diastolic phase, compromising its ability to fill with blood. This diastolic abnormality creates a clinical presentation remarkably similar to HFpEF caused by other etiologies, particularly in older adults, where the prevalence of both conditions rises sharply. Indeed, approximately 25% of patients over 85 years have the acquired variant of transthyretin amyloidosis (ATTRwt), with 5-10% showing cardiac involvement.
The diagnostic challenge intensifies with comorbidity profiles. Patients with cardiac amyloidosis carry substantial pre-existing conditions at diagnosis, including hypertension (78.9%), ischemic heart disease (55.9%), atrial fibrillation (72.8%), and chronic kidney disease (63.4%). These comorbidities, common in the general HFpEF population, further obscure the underlying amyloid aetiology.
This overlap creates a critical diagnostic imperative. Without proper treatment, the average survival time after being diagnosed with ATTR cardiac amyloidosis is only 2.5 to 3.6 years for hereditary and wild types, respectively. Recognizing unique characteristics that may indicate cardiac amyloidosis in the HFpEF population is crucial for enhancing outcomes in this complex patient group.
Red Flag Indicators for Suspected Cardiac Amyloidosis
Recognizing distinct warning signs apart from typical heart failure symptoms facilitates the earlier diagnosis of cardiac amyloidosis. These “red flags” often come before the onset of obvious amyloid heart disease, sometimes by a few years, giving doctors a key chance to step in. For early identification, doctors need to keep a high level of suspicion, especially in patients who have heart failure symptoms and other symptoms that don’t seem to be related to the heart.
Carpal Tunnel Syndrome and Peripheral Neuropathy
Carpal tunnel syndrome (CTS) represents the most common early marker of underlying amyloid heart disease. In patients with wild-type transthyretin amyloidosis (ATTRwt) cardiomyopathy, CTS affects up to 88% of cases compared to merely 7% of age-matched controls. This association proves even more striking in bilateral cases—approximately 4% of all men with bilateral CTS harbor ATTRwt cardiomyopathy, rising to 33% when left ventricular hypertrophy coexists.
What makes CTS particularly valuable as a clinical indicator is its temporal relationship to cardiac symptoms. CTS typically predates cardiac manifestations by 6-10 years in hereditary transthyretin amyloidosis. Furthermore, several characteristics distinguish amyloid-related CTS:
- Frequently bilateral presentation
- Relatively aggressive progression
- Higher recurrence rates following surgical release compared to non-amyloid CTS
Peripheral neuropathy likewise serves as a cardinal sign, manifesting in approximately 80% of patients with hereditary transthyretin amyloidosis as their initial symptom. It typically presents as a symmetrical, length-dependent polyneuropathy with prominent small fiber involvement, causing neuropathic pain and sensory disturbances. Although less common in wild-type variants, its presence alongside other red flags substantially increases diagnostic probability.
Unintentional Weight Loss and Orthostatic Hypotension
Orthostatic hypotension (OH)—defined as a sustained decrease of at least 20 mmHg in systolic blood pressure or 10 mmHg in diastolic blood pressure within three minutes of standing—emerges as another distinctive marker of amyloid heart disease. This autonomic disturbance occurs relatively early in the disease course, affecting approximately 11.7% of patients at enrollment in clinical studies—exceeding the prevalence of diarrhea (2.4%) or unintentional weight loss (3.1%).
The clinical impact of OH extends beyond mere numbers. Patients experiencing this symptom report significantly worse quality of life. Many describe dizziness, lightheadedness, or syncope that substantially hinders daily activities; in severe cases, three out of 11 patients in one study became completely incapacitated by this symptom alone.
Concurrent unintentional weight loss warrants particular attention, especially when combined with gastrointestinal disturbances. All patients in one series demonstrated substantial weight reduction, whereas another reported a weight loss of 10 kg over one year in a typical case. The combination of OH and weight loss often leads to misdiagnosis as adrenal insufficiency, potentially delaying appropriate treatment.
History of Atrial Fibrillation or Biceps Tendon Rupture
Atrial fibrillation represents another red flag, particularly when it occurs alongside other suggestive findings. Although often attributed to standard cardiovascular disease, its presence in conjunction with unexplained left ventricular wall thickness should trigger consideration of amyloid heart disease. This arrhythmia commonly occurs in cardiac amyloidosis patients who demonstrate intolerance to standard antihypertensive medications.
Perhaps the most overlooked musculoskeletal indicator involves spontaneous tendon ruptures, especially affecting the distal biceps. Recent evidence establishes a compelling association between ruptured distal biceps tendons and wild-type transthyretin cardiac amyloidosis. This connection stems from amyloid deposition in various ligaments and tendons, creating structural weakness.
The constellation of these extracardiac manifestations alongside heart failure symptoms substantially increases the probability of underlying amyloid heart disease. Clinicians evaluating patients with preserved ejection fraction heart failure should routinely assess for:
- History of bilateral carpal tunnel syndrome
- Peripheral neuropathy symptoms
- Unexplained weight loss with orthostatic hypotension
- Atrial fibrillation with diminishing antihypertensive tolerance
- Previous tendon ruptures, especially the biceps
Overall, these red flags offer valuable diagnostic clues that, when appropriately recognized, facilitate earlier identification of this frequently missed condition.
Functional Status and Quality of Life in Suspected Cases
Patient assessment in cardiac amyloidosis extends beyond identifying diagnostic red flags to evaluating functional status and quality of life. These metrics provide crucial insights into disease severity, progression, and treatment response in amyloid heart disease.
NYHA Functional Class Distribution in Amyloid Heart Failure
The New York Heart Association (NYHA) functional classification is a key part of figuring out how bad the symptoms are for people with amyloid heart failure. In various studies, nearly 50% of patients with transthyretin amyloidosis cardiomyopathy (ATTR-CA) exhibit advanced NYHA class symptoms at the time of diagnosis. Specifically, 50.9% of ATTR-CA patients demonstrate NYHA class ≥III at initial evaluation, indicating marked limitations in physical activity with symptoms occurring during minimal exertion.
The distribution across NYHA classes varies by heart failure phenotype. Among patients with ATTR-CA, the breakdown typically shows:
- Class I: 11% (minimal symptoms)
- Class II: 38.2% (mild symptoms with ordinary activity)
- Class III: 46.2% (moderate symptoms with less than ordinary activity)
- Class IV: 4.7% (severe symptoms at rest)
Notably, patients with reduced ejection fraction demonstrate worse symptom burden, with 71.7% presenting in NYHA class ≥III versus 42.9% in those with preserved ejection fraction. It suggests that, counterintuitively, preserved ejection fraction cases may present with less severe functional limitations initially, potentially contributing to diagnostic delays.
Functional status profoundly impacts survival in amyloid heart disease. In a study of 167 ATTR-CA patients (94.6% wild-type), those presenting with NYHA class III showed substantially worse outcomes than those in NYHA class I/II. The proportion of patients who died during follow-up was markedly higher in the NYHA III group (25.0% versus 5.6%). Similarly, major adverse cardiac events occurred in 56.7% of NYHA III patients compared to 20.6% in NYHA I/II patients.
This prognostic impact persists even after statistical adjustment. Baseline NYHA class III status was associated with an adjusted hazard ratio of 5.85 for all-cause death. Hence, functional class assessment serves as both a clinical and prognostic tool in amyloid heart failure management.
KCCQ-12 Scores in Suspected vs. Typical HFpEF Patients
The Kansas City Cardiomyopathy Questionnaire (KCCQ-12) offers a more comprehensive assessment of heart failure-related health status. This validated 12-item instrument measures physical limitations, symptoms, quality of life, and social limitations on a 0-100 scale, with higher scores indicating better health status.
In amyloid heart disease, KCCQ-12 scores typically fall in the “fair” range (score of approximately 50), revealing substantial impairment across multiple domains. However, the impact varies by amyloidosis type. Patients with light chain (AL) amyloidosis demonstrate stronger associations between poor KCCQ scores and adverse outcomes than those with ATTR amyloidosis. Specifically, AL amyloidosis patients in the lowest KCCQ quartile (<25) showed a hazard ratio of 7.54 for heart failure hospitalization or death, versus 1.42 in ATTR patients with similarly low scores.
When comparing amyloid heart failure to typical HFpEF, several differences emerge. Both conditions show strong correlations between KCCQ scores and NYHA class (r = -0.61 for HFpEF, r = -0.54 for HFrEF), yet the prognostic implications differ. Among HFpEF patients with suspected amyloidosis, those with the lowest KCCQ quartile demonstrate substantially worse one-year event-free survival (13.8%) compared to those in the highest quartile (77.8%).
Treatment interventions influence quality of life metrics differently in amyloid cardiomyopathy versus general HFpEF. In clinical trials of tafamidis for ATTR cardiomyopathy, patients receiving active treatment were 2.5 times more likely to experience meaningful improvement in KCCQ-OS scores than those receiving placebo. This effect persisted across all NYHA classes, essentially challenging the traditional notion that advanced functional limitation precludes quality of life improvement.
Evidently, both the NYHA classification and the KCCQ scores provide complementary information in suspected cardiac amyloidosis cases. Together, they offer a multi-dimensional assessment of disease burden that extends beyond conventional diagnostic parameters, facilitating more personalized management approaches for this often-overlooked cause of heart failure with preserved ejection fraction.
Demographic Patterns in Symptom Burden
Demographic factors profoundly influence both the presentation and recognition of amyloid heart disease, creating distinct patterns that merit careful consideration in clinical practice.
Gender Differences in Red Flag Symptom Reporting
Wild-type transthyretin amyloidosis cardiomyopathy (ATTRwt-CA) demonstrates a striking male predominance. In several cohort studies, men constituted over 80% of cases. Meanwhile, in the pivotal ATTR-ACT trial, an astounding 96% of enrolled participants were male. Female patients, conversely, tend to present at considerably older ages—one study reported a mean age of 82.9 years for women versus 77.1 years for men.
The clinical manifestations differ substantially between sexes. Women typically present with a more advanced New York Heart Association (NYHA) functional class (2.6 ± 0.8 vs. 2.2 ± 0.7 in men). Interestingly, in a cohort analysis by Takashio et al., women were more frequently classified as NYHA class III (57% vs. 35%) or IV (7% vs. 1%) compared to men. Furthermore, women exhibit higher B-type natriuretic peptide levels (394 pg/mL vs. 236 pg/mL) and frequently present with a history of heart failure with preserved ejection fraction.
Anatomically, female patients typically display smaller left ventricular chambers (37.3 ± 5.2 mm vs. 42.3 ± 6.3 mm) with thinner interventricular septum (14.1 ± 2.5 mm vs. 15.7 ± 2.6 mm). Remarkably, women demonstrate a much higher prevalence of concurrent moderate-to-severe aortic stenosis (45% vs. 5%), potentially complicating diagnosis.
Age-Related Decline in Functional Status
The age distribution of ATTRwt amyloidosis reveals distinct patterns—approximately 13.7% of patients are under 70 years, 49.1% between 70-79 years, 34.5% between 80-89 years, and 2.8% aged 90 or above. As age advances, functional status deteriorates precipitously. The proportion of patients with Karnofsky Performance Status scores ≤70 increases from 17.1% in those under 70 years to 46.1% in the 80-89 age group.
Diagnostic delays vary by age cohort. The median time from symptom onset to diagnosis ranges from 1.7 years in patients under 70 to 0.7 years in those over 90. Notably, the greatest diagnostic delay occurs in women under 70 years, reaching approximately 5 years—a concerning finding given the progressive nature of the disease.
Family History Awareness and Diagnostic Gaps
Family history awareness remains critically important, as up to 60% of individuals with a family history of amyloidosis eventually develop the condition themselves. Nevertheless, substantial diagnostic gaps persist, particularly in underrepresented populations.
Racial disparities in amyloid heart disease are pronounced. Black patients have a 2.5-fold increased risk of heart failure hospitalization compared to White patients. In particular, the Val122Ile mutation—observed almost exclusively in Black patients—results in predominantly cardiac involvement with shorter median survival (25.6 months vs. 43 months in ATTRwt).
Black patients with ATTR cardiomyopathy typically present with worse clinical status, including higher rates of prior heart failure hospitalizations (49% vs. 26%), more severe NYHA functional class III symptoms (42% vs. 21%), and significantly worse renal function (eGFR 53.8 ± 20.8 vs. 66.1 ± 19.2 mL/min/1.73 m²). Their hemodynamic parameters are uniformly worse, encompassing higher filling pressures, lower cardiac output, and elevated pulmonary vascular resistance.
For most patients with ATTR cardiomyopathy, the median time from cardiac symptom onset to diagnosis remains approximately 2-3 years—an interval that must be shortened through improved awareness and screening initiatives.
Symptom-Based Screening Tools in Clinical Practice
Effective identification of cardiac amyloidosis within heart failure populations requires systematic screening approaches. Current diagnostic delays—frequently exceeding 4 years from symptom onset—necessitate practical tools that can be readily deployed in clinical settings.
Design of the 7-Item Red Flag Checklist
A structured questionnaire approach forms the cornerstone of early screening efforts. The self-created 7-item red flag checklist serves as an exploratory tool to identify potential cases of amyloid heart disease among patients with preserved ejection fraction heart failure. This instrument consists of simple yes/no questions addressing the most frequently reported symptoms and clinical indicators. Questions target key warning signs such as unintentional weight loss and peripheral neuropathy symptoms (numbness, tingling, or burning sensations in extremities). Upon completion, patients scoring 0-1 points are classified as typical HFpEF cases, whereas those with two or more positive responses warrant consideration for suspected cardiac amyloidosis.
Limitations of Self-Reported Screening
In practice, even well-designed screening protocols face substantial implementation challenges. Among patients identified as high-risk (those with bilateral carpal tunnel syndrome, atrial fibrillation, and left ventricular hypertrophy on echocardiography), merely 13.4% underwent appropriate assessment for cardiac amyloidosis. Of these evaluated patients, only three received technetium-99m pyrophosphate scans—a critical diagnostic tool with remarkably high sensitivity and specificity for transthyretin cardiac amyloidosis. As a result, substantial diagnostic opportunities remain missed, as evidenced by the considerably higher mortality rate (43.3% vs. 24.6%) observed in undiagnosed high-risk groups.
Potential for Early Referral and Imaging Follow-Up
Emerging protocols showcase promising approaches for earlier identification. At a minimum, experts recommend screening all men over 50 and women over 60 presenting with bilateral carpal tunnel syndrome through routine flexor tenosynovial biopsies during carpal tunnel release procedures. This approach capitalizes on the fact that carpal tunnel manifestations often precede cardiac symptoms by 7-10 years. Upon positive biopsy findings, immediate cardiology referral enables a comprehensive workup before cardiac symptoms develop.
Cardiologists must consider several key indicators when evaluating potential cases, primarily looking for unexplained left ventricular wall thickening, arrhythmias (chiefly atrial fibrillation), and progressive intolerance to antihypertensive medications. The initial diagnostic step involves monoclonal protein screening to rule out light chain amyloidosis, followed by bone scintigraphy when appropriate. Remarkably, this latter technique can diagnose transthyretin cardiac amyloidosis with approximately 100% accuracy when properly utilized.
Technological advances offer additional screening possibilities, including AI-enhanced echocardiography that identifies amyloid heart disease from standard video clips with impressive accuracy (sensitivity 85%, specificity 93%).
Statistical Correlations and Predictive Modeling
Statistical analysis provides critical insights into cardiac amyloid disease patterns and aids in clinical decision-making. Understanding these relationships enables practitioners to make informed diagnostic choices based on quantifiable data.
Spearman Correlation Between Red Flags and NYHA Class
Quantitative analysis reveals compelling associations between symptom burden and clinical outcomes in suspected amyloid heart failure cases. The red flag burden demonstrates a positive correlation with worse functional status (r = 0.26, p < 0.01). Correspondingly, this burden shows a stronger negative correlation with quality of life metrics (r = -0.32, p < 0.01). These relationships persist across demographic subgroups, underscoring the clinical utility of red flag screening regardless of patient characteristics.
Ordinal Logistic Regression for Diagnostic Group Prediction
Multivariate modeling confirms that clinical parameters effectively predict amyloidosis probability. Ordinal logistic regression reveals that poorer functional status substantially increases suspected cardiac amyloidosis risk (B = 1.950, p < 0.001). NYHA functional class likewise exhibits substantial predictive power (B = 0.580, p < 0.001). Concurrently, quality of life scores show a negative coefficient (B = -0.065, p = 0.002), indicating that diminished quality of life correlates with increased likelihood of amyloid heart disease. The model explains 26.8% of variance (pseudo-R² = 0.268) with strong statistical validity (χ² = 46.32, p < 0.001).
Conclusion 
Cardiac amyloidosis represents a critical yet frequently overlooked etiology of heart failure with preserved ejection fraction. Throughout this review, we have demonstrated how amyloid heart disease masquerades as typical HFpEF, delaying diagnosis and appropriate management for countless patients. This diagnostic challenge stems from substantial symptom overlap, yet careful attention to red flag indicators can markedly improve early detection rates.
The constellation of extracardiac manifestations—notably bilateral carpal tunnel syndrome, peripheral neuropathy, orthostatic hypotension, and unexplained weight loss—serves as a vital clinical roadmap for practitioners. These warning signs typically precede overt cardiac symptoms by several years, offering a crucial window for intervention. Accordingly, systematic screening approaches targeting these red flags could revolutionize current practice patterns and patient outcomes.
Functional status assessment remains fundamental to patient evaluation. NYHA classification distribution reveals approximately half of ATTR-CA patients present with class ≥III symptoms at diagnosis, while KCCQ-12 scores typically fall in the “fair” range. These metrics not only quantify disease burden but also provide prognostic information, as demonstrated by the substantially worse outcomes observed in patients with advanced functional limitations.
Demographic factors undoubtedly influence disease presentation and recognition. Male predominance characterizes ATTRwt cardiomyopathy, though women typically exhibit more advanced symptoms at presentation despite thinner ventricular walls. Age-related functional decline correlates with worse outcomes, while racial disparities persist—Black patients face higher risk, earlier onset, and more severe manifestations than their White counterparts.
Structured screening tools offer practical solutions for earlier identification. The 7-item red flag checklist offers an accessible structure for risk stratification, but difficulties with implementation are still common. Statistical analysis substantiates robust correlations among red flag burden, functional status, and quality of life metrics, thereby affirming the clinical efficacy of these screening
methodologies.
When medical professionals are checking on patients with preserved ejection fraction heart failure, they need to be extra careful about this condition. The diagnostic pathway now includes non-invasive methods like bone scintigraphy, which is almost always accurate when done correctly. Also, new technologies like AI-enhanced echocardiography promise to make screening even more efficient.
Cardiac amyloidosis illustrates how ostensibly typical clinical manifestations can obscure intricate underlying pathologies. Consequently, a thorough evaluation of HFpEF patients should consistently include the assessment for red flag indicators, especially within high-risk demographic groups. This method will reduce the time it takes to make a diagnosis, allow for earlier treatment, and improve results for this difficult group of patients.
Key Takeaways
Cardiac amyloidosis frequently masquerades as typical heart failure with preserved ejection fraction, creating a critical diagnostic blind spot that affects patient outcomes and treatment decisions.
- Red flag symptoms precede cardiac manifestations by years: Bilateral carpal tunnel syndrome, peripheral neuropathy, and orthostatic hypotension often appear 6-10 years before heart failure symptoms develop.
- Only 10% of practitioners systematically screen for cardiac amyloidosis in HFpEF patients, despite two-thirds reporting diagnostic experience with the condition.
- Male patients over 80 face the highest risk, while women present with more advanced symptoms despite later diagnosis, creating gender-specific screening considerations.
- Simple 7-item screening checklists can identify high-risk patients for further cardiac evaluation, potentially reducing diagnostic delays from 2-3 years to months.
- Bone scintigraphy achieves nearly 100% diagnostic accuracy for transthyretin cardiac amyloidosis when properly utilized, eliminating the need for invasive cardiac biopsy.
Early recognition of these warning signs could transform outcomes for thousands of patients currently misdiagnosed with typical HFpEF, enabling access to targeted therapies that can slow disease progression and improve quality of life.
Frequently Asked Questions:
FAQs
Q1. What are the key symptoms that differentiate cardiac amyloidosis from typical heart failure? While symptoms like fatigue and shortness of breath are common in both conditions, cardiac amyloidosis often presents with additional red flags such as carpal tunnel syndrome, peripheral neuropathy, unexplained weight loss, and orthostatic hypotension. These symptoms may precede cardiac issues by several years.
Q2. How common is cardiac amyloidosis in patients diagnosed with heart failure with preserved ejection fraction (HFpEF)? Cardiac amyloidosis is frequently underdiagnosed in HFpEF patients. Studies suggest that a significant portion of HFpEF cases, particularly in older adults, may be due to unrecognized cardiac amyloidosis. However, only about 10% of medical practitioners routinely screen for it.
Q3. Are there gender differences in the presentation of cardiac amyloidosis? Yes, there are notable gender differences. Wild-type transthyretin amyloidosis cardiomyopathy shows a strong male predominance, with over 80% of cases occurring in men. However, women typically present at older ages and often with more advanced symptoms despite having thinner ventricular walls.
Q4. What diagnostic tools are most effective for identifying cardiac amyloidosis? Non-invasive techniques like bone scintigraphy have shown nearly 100% accuracy in diagnosing transthyretin cardiac amyloidosis when properly used. Additionally, a 7-item red flag checklist can help identify high-risk patients who should undergo further cardiac evaluation.
Q5. How does early detection of cardiac amyloidosis impact patient outcomes? Early detection of cardiac amyloidosis is crucial for improving patient outcomes. It allows for the timely initiation of targeted therapies that can slow disease progression and improve quality of life. Currently, diagnostic delays often exceed 2-3 years from symptom onset, highlighting the importance of improved screening and awareness.
References:
[1] – https://www.journal-of-cardiology.com/article/S0914-5087(21)00278-1/fulltext
[3] – https://onlinelibrary.wiley.com/doi/full/10.1002/clc.23985
[4] – https://pmc.ncbi.nlm.nih.gov/articles/PMC8916707/
[5] – https://www.ahajournals.org/doi/10.1161/JAHA.123.033478
[6] – https://pmc.ncbi.nlm.nih.gov/articles/PMC9872973/
[7] – https://pmc.ncbi.nlm.nih.gov/articles/PMC10833096/
[8] – https://www.frontiersin.org/journals/neurology/articles/10.3389/
fneur.2021.707134/full
[9] – https://pmc.ncbi.nlm.nih.gov/articles/PMC6763509/
[10] – https://www.sciencedirect.com/science/article/pii/S1566070219301043
[11] – https://www.ahajournals.org/doi/10.1161/01.CIR.34.5.883
[13] – https://www.acpjournals.org/doi/10.7326/aimcc.2023.1130
[14] – https://pmc.ncbi.nlm.nih.gov/articles/PMC11631306/
[15] – https://pmc.ncbi.nlm.nih.gov/articles/PMC12040782/
[16] – https://www.sciencedirect.com/science/article/pii/S1071916423008400
[17] – https://pmc.ncbi.nlm.nih.gov/articles/PMC4545244/
[18] – https://jamanetwork.com/journals/jamacardiology/fullarticle/2801004
[19] – https://pmc.ncbi.nlm.nih.gov/articles/PMC10942898/
[20] – https://www.sciencedirect.com/science/article/pii/S2772963X24002801
[21] – https://pmc.ncbi.nlm.nih.gov/articles/PMC11229544/
[22] – https://www.rarediseaseadvisor.com/news/red-flags-cardiac-amyloidosis-missed/
[24] – https://pmc.ncbi.nlm.nih.gov/articles/PMC11305997/
[26] – https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.123.064538

