Testosterone Replacement Therapy: New Guidelines Challenge Old Beliefs
Introduction
Testosterone replacement therapy (TRT) usage has risen sharply over the past two decades, with rates tripling across the United States. By 2013, an estimated 2.3 million American men were receiving this treatment, reflecting both increased awareness of hypogonadism and evolving patient and physician attitudes toward hormonal therapy. The reported prevalence of male hypogonadism varies widely, ranging from 2 percent to 39 percent depending on the diagnostic criteria applied, with several population-based studies estimating rates near 12 percent. Despite these substantial figures, current prescription practices highlight ongoing concerns about adherence to clinical guidelines. In the United States, approximately 60 percent of testosterone therapy prescriptions are written by primary care physicians, and data from Nova Scotia, Canada, indicate that this proportion averages as high as 92 percent.
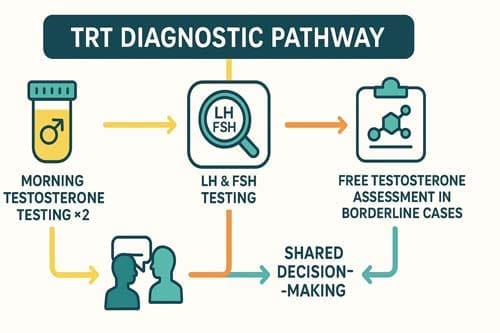
A critical issue is that many prescriptions are initiated without comprehensive diagnostic evaluation. Evidence suggests that up to one-quarter of men who begin testosterone therapy do so without having their serum testosterone levels measured. This practice diverges from evidence-based recommendations, which emphasize confirmation of biochemical deficiency through at least two separate early morning testosterone measurements, in conjunction with the presence of consistent clinical symptoms. Failure to establish an accurate diagnosis risks both overtreatment and underrecognition of underlying conditions contributing to androgen deficiency.
The clinical consequences of untreated hypogonadism are significant. Low testosterone levels have been associated with reduced bone mineral density, predisposing patients to osteoporosis and fracture. They also contribute to adverse metabolic outcomes, including insulin resistance and type 2 diabetes mellitus, and may play a role in cardiovascular disease development. Beyond physical health, hypogonadism is linked to decreased libido, impaired mood, fatigue, and cognitive decline, all of which negatively affect quality of life and functional capacity. At the same time, inappropriate initiation of TRT in men without true hypogonadism exposes patients to unnecessary risks such as erythrocytosis, prostate enlargement, infertility, and potential cardiovascular complications, while also imposing considerable costs on healthcare systems.
In light of these challenges, several professional organizations have issued updated clinical practice guidelines to clarify the diagnostic thresholds, therapeutic indications, and monitoring requirements for TRT. These guidelines stress the importance of individualized assessment, shared decision-making, and long-term safety surveillance. Recent evidence has challenged earlier assumptions regarding treatment thresholds, particularly the absolute testosterone levels at which therapy should be considered, as well as the balance of benefits and risks across different patient populations.
This article reviews the evolving perspectives on testosterone replacement therapy, examining current evidence from epidemiological studies, clinical trials, and consensus guidelines. It aims to provide graduate-level practitioners with a comprehensive framework for evaluating candidates for TRT, initiating therapy when appropriate, and implementing structured follow-up and monitoring to ensure both efficacy and safety. By integrating new evidence with established practice standards, clinicians can optimize patient outcomes while minimizing the risks associated with inappropriate or poorly monitored therapy.

Updated Diagnostic Criteria for Male Hypogonadism
The diagnosis of male hypogonadism has evolved substantially, as professional societies seek to establish more precise criteria that balance scientific evidence with individual patient factors. These updates aim to standardize clinical practice while ensuring that the unique presentations of each patient are appropriately considered.
Morning total testosterone threshold: 264–300 ng/dL
The definition of biochemical testosterone deficiency varies across international guidelines, reflecting ongoing debate and evolving evidence. The American Urological Association (AUA) recommends a cutoff of 300 ng/dL (10.4 nmol/L) for the diagnosis of hypogonadism, whereas the Endocrine Society suggests a slightly lower threshold of 264 ng/dL (9.2 nmol/L). Other professional bodies propose different benchmarks: the American Association of Clinical Endocrinologists advises a threshold of 200 ng/dL (6.9 nmol/L), and the International Society of Andrology recommends 230 ng/dL (8.0 nmol/L). The Australian guidelines take a more individualized approach by using age-adjusted reference ranges, with the lower limit set at >10.4 nmol/L (approximately 300 ng/dL) for men under 35 years and declining progressively with age to >6.4 nmol/L (185 ng/dL) for men over 70. These variations highlight the lack of universal consensus and emphasize the importance of integrating clinical judgment with biochemical assessment.
Role of free testosterone and SHBG in borderline cases
In men with borderline total testosterone levels or suspected abnormalities in testosterone binding, assessing free testosterone and SHBG is particularly valuable. SHBG strongly influences the fraction of circulating testosterone that is biologically active. Research indicates that up to 20% of men with total testosterone levels above 300 ng/dL may still have low bioavailable testosterone, defined as <156 ng/dL (5.4 nmol/L), underscoring the limitations of total testosterone alone. Both the British Society for Sexual Medicine and the International Society for Sexual Medicine recommend measuring LH, SHBG, and prolactin in symptomatic men with testosterone levels in the intermediate range of 8–12 nmol/L (230–346 ng/dL). These additional markers help clarify the diagnosis, particularly in men whose clinical symptoms are not explained by total testosterone alone.
Two-sample confirmation and fasting state requirement
Most guidelines stress the need for at least two morning measurements of total testosterone to confirm the diagnosis of hypogonadism, given the diurnal variation in hormone secretion and intraindividual variability. The AUA advises that if the first measurement is <300 ng/dL and the second result falls within the normal range, a third test may be considered based on clinical judgment. Historically, fasting samples were recommended, as glucose and insulin levels can affect SHBG concentrations. However, recent evidence suggests that fasting may not be strictly necessary. A large study comparing fasting and non-fasting samples demonstrated no statistically significant difference in testosterone values, supporting a more flexible approach while still maintaining diagnostic accuracy.
Differentiating primary vs secondary hypogonadism using LH and FSH
Measuring luteinizing hormone (LH) and follicle-stimulating hormone (FSH) helps distinguish the origin of hypogonadism. Primary hypogonadism (testicular failure) presents with low testosterone and elevated LH/FSH levels. Conversely, secondary hypogonadism (hypothalamic-pituitary dysfunction) shows low or inappropriately normal LH/FSH levels alongside low testosterone. This distinction guides treatment approaches and identifies patients requiring further evaluation for pituitary disorders.
New Treatment Guidelines and Individualized Therapy
Current treatment approaches for male hypogonadism emphasize individualized therapy based on patient-specific factors and shared clinical decision-making. These updated guidelines reflect evolving understanding of testosterone’s therapeutic effects and safety profile.
Testosterone therapy goals: symptom relief and mid-normal levels
The primary goal of testosterone replacement therapy is improving symptoms while minimizing potential adverse events. Guidelines recommend targeting testosterone concentrations in the mid-normal range, regardless of administration route. Sexual symptoms typically improve within 6 weeks of starting treatment, whereas other benefits may take up to 12 months to manifest. Regular monitoring ensures that testosterone levels reach the targeted range (approximately 3-5 ng/mL) while evaluating symptom improvement.
Shared decision-making in borderline testosterone cases
Patient factors play a crucial role in testosterone prescribing decisions. For men with borderline testosterone levels, clinicians should engage patients in shared decision-making after thorough counseling about potential benefits and risks. Accordingly, the AUA recommends that clinicians use their clinical judgment for men with total testosterone levels >300 ng/dL who remain highly symptomatic. Moreover, identifying patient-specific goals before initiating therapy allows for better assessment of treatment success.
Lifestyle modification as first-line in functional hypogonadism
Both the Endocrine Society and Australian guidelines recommend lifestyle modifications as first-line interventions for functional hypogonadism before initiating testosterone therapy. Weight loss and physical exercise have demonstrated effectiveness in increasing testosterone levels and improving sexual function. Specifically, adults need 150-300 minutes of moderate-intensity aerobic activity weekly, with benefits proportional to exercise duration and intensity. Furthermore, a short-term testosterone trial might help obese hypogonadal patients overcome inactivity and prepare for lifestyle changes.
Guideline differences: AUA vs Endocrine Society vs EAU
Though professional guidelines share common ground, they differ in several key aspects. The Endocrine Society applies a lower testosterone threshold (264 ng/dL) compared to the AUA (300 ng/dL). Regarding cardiovascular safety, the AUA recommends against testosterone therapy in men with major adverse cardiovascular events within 3-6 months, whereas the EAU does not specify a timeframe but lists acute cardiovascular events as relative contraindications. Additionally, the EAU recommends considering echocardiography before initiating testosterone in men with pre-existing cardiovascular disease.
Comparing Testosterone Formulations and Their Clinical Use
Selecting the appropriate testosterone formulation requires careful consideration of pharmacokinetics, patient preference, and safety profiles. Each delivery method offers distinct advantages and limitations for clinical practice.
Transdermal gels vs intramuscular injections: adherence and absorption
Testosterone formulations differ substantially in their absorption patterns and pharmacokinetic profiles. Intramuscular (IM) injections create pronounced peaks of supranormal testosterone levels that gradually decline until subsequent administration, resulting in substantial fluctuations. In contrast, transdermal gels produce subtle, short-term (24-48 hours) increases with daily application maintaining more consistent levels. Free testosterone peak-trough fluctuations measured 26.7 ± 12.8 pg/mL with IM testosterone versus only 2.7 ± 10.7 pg/mL with gel formulations. Beyond pharmacokinetics, clinical outcomes also differ – compared with gel users, injection initiators demonstrated higher hazards of cardiovascular events (1.26), hospitalization (1.16), and death (1.34).
Intranasal testosterone for fertility preservation
Intranasal testosterone (Natesto) offers a unique option for men concerned about fertility preservation. During clinical trials, mean testosterone increased from 230(62) to 605(278) ng/dL while semen parameters remained largely unchanged (sperm concentration 26.6(15.2) vs 26.0(21.2) million/cc). Only 7.9% of men developed severe oligospermia and 2.6% became azoospermic – all recovered after discontinuation. This preservation of spermatogenesis occurs because the short half-life (10-100 minutes) allows partial maintenance of gonadotropin pulsatility. After 6 months of therapy, 93.9% of men maintained total motile sperm counts above 5 million.
Oral testosterone undecanoate: pharmacokinetics and boxed warnings
Oral testosterone undecanoate (TU) avoids first-pass metabolism through intestinal lymphatic absorption (90-100%), with peak serum levels occurring 3-5 hours post-administration. Unlike earlier methyltestosterone formulations associated with hepatotoxicity, TU demonstrates minimal liver impact. Nevertheless, oral TU carries a boxed warning for blood pressure elevations that may increase cardiovascular risk – studies showed mean systolic increases of 4.9 ± 8.7 mmHg versus 0.2 ± 9.4 mmHg with topical formulations. Newer self-emulsifying delivery systems improve absorption without requiring high-fat meals.
Subdermal pellets and SC injections: long-acting options
Testosterone pellets implanted subdermally provide sustained release over 2-6 months, typically requiring replacement every 3 months. Main advantages include guaranteed compliance and infrequent dosing, yet drawbacks include surgical insertion, cost, and potential extrusion (10% incidence). Subcutaneous (SC) injections represent an emerging alternative to traditional IM administration, offering similar pharmacokinetic profiles with reduced discomfort. Weekly SC testosterone enanthate/cypionate at individualized doses (50-150 mg) successfully achieved target serum levels in transgender men regardless of BMI.
Formulation-specific risks: polycythemia, BP elevation, transference
Each testosterone formulation carries unique risk considerations. Polycythemia occurs most frequently with IM injections – European guidelines contraindicate testosterone at hematocrit greater than 54%, whereas American guidelines use 50% as the threshold. Transdermal formulations may reduce erythrocytosis risk for patients requiring ongoing treatment. Topical preparations carry risk of unintended transfer to women and children, prompting FDA boxed warnings. Meanwhile, oral formulations raise concerns regarding blood pressure elevations and lipid changes. Regular monitoring remains essential regardless of formulation choice.
Monitoring Protocols and Safety Considerations
Careful monitoring is a cornerstone of safe and effective testosterone replacement therapy (TRT). Regular clinical evaluations and laboratory assessments allow for early detection of adverse effects and ensure that therapeutic benefits outweigh potential risks. Monitoring should be individualized but must adhere to evidence-based guidelines and standardized intervals to maintain both safety and efficacy.
Hematocrit Monitoring
Hematocrit assessment is mandatory before initiating TRT to establish a baseline. Follow-up measurements are recommended at 3 months, 6 months, and 12 months after therapy initiation, and annually thereafter if stable. Elevated hematocrit represents one of the most important hematological risks of TRT due to its association with increased blood viscosity and potential thromboembolic events. Clinical guidelines differ regarding threshold values. In the United States, testosterone therapy is contraindicated when hematocrit exceeds 50 percent, while European guidelines use 54 percent as the upper limit. Once hematocrit rises above 54 percent, management options include discontinuing therapy or performing therapeutic phlebotomy. After hematocrit levels decline below 50 percent and secondary causes of erythrocytosis are excluded, TRT may be resumed at a reduced dose to minimize recurrence.
Prostate Surveillance
Given concerns regarding the potential impact of TRT on prostate health, baseline evaluation should include both prostate-specific antigen (PSA) testing and a digital rectal examination (DRE). PSA levels should be reassessed at 3 months, 12 months, and then annually if stable. Clinicians should be alert to vital changes in PSA values. A confirmed increase of more than 1.4 ng/mL within 12 months or an absolute PSA level exceeding 4.0 ng/mL warrants referral to a urologist for further evaluation. Data indicate that the 95th percentile for PSA increases at 3 months and 12 months are 1.2 ng/mL and 1.7 ng/mL, respectively, underscoring the importance of ongoing surveillance.
Cardiovascular Safety
Cardiovascular risk remains a key consideration in TRT. The TRAVERSE trial provided important evidence, demonstrating that testosterone therapy does not increase overall cardiovascular risk compared with placebo. Based on these findings, the U.S. Food and Drug Administration (FDA) removed prior boxed warning language regarding cardiovascular outcomes. Nevertheless, the trial identified higher incidences of pulmonary embolism, atrial fibrillation, and acute kidney injury in the testosterone group compared with placebo. These findings highlight the need for careful patient selection, risk stratification, and continued vigilance during treatment.
Criteria for Discontinuation
Treatment continuation should be reassessed regularly, with particular attention to symptomatic improvement. If patients show no meaningful improvement in symptoms after 3 to 6 months of therapy despite normalization of testosterone levels, discontinuation should be strongly considered. Shared decision-making is essential in this context, ensuring patients understand that the absence of clinical benefit may outweigh the risks of ongoing therapy. Periodic reevaluation thereafter helps confirm that continued TRT remains appropriate and beneficial.

Conclusion 
Conclusion
Testosterone replacement therapy has undergone substantial clinical reevaluation in recent years. Previously held beliefs about diagnostic thresholds, treatment approaches, and safety monitoring have evolved into more nuanced, evidence-based guidelines. Consequently, clinicians must adapt their practices to reflect these advancements.
The diagnostic criteria for male hypogonadism now involve more comprehensive evaluation beyond simple testosterone cutoffs. Specifically, practitioners should obtain at least two morning testosterone measurements, consider age-adjusted reference ranges when appropriate, and properly differentiate between primary and secondary hypogonadism through LH and FSH testing. Additionally, free testosterone and SHBG measurements provide crucial diagnostic clarity in borderline cases.
Treatment approaches have likewise shifted toward greater personalization. Rather than applying universal protocols, clinicians should engage patients in shared decision-making, especially for those with borderline testosterone levels. Lifestyle modifications remain first-line interventions for functional hypogonadism, though short-term testosterone trials may help overcome initial barriers to physical activity. Above all, treatment goals should focus on symptom relief while maintaining testosterone levels in the mid-normal range.
The selection of testosterone formulations demands careful consideration of individual patient factors. Each delivery method presents distinct advantages – transdermal gels offer stable hormone levels, intranasal formulations preserve fertility, and long-acting options enhance compliance. However, these benefits must be weighed against formulation-specific risks including polycythemia, blood pressure elevation, and unintended transfer.
Safety monitoring throughout therapy remains essential regardless of formulation choice. Regular assessment of hematocrit, PSA levels, and cardiovascular parameters allows for timely intervention when necessary. Nevertheless, recent evidence challenges previous concerns about cardiovascular risk, though vigilance remains warranted for specific adverse events.
The landscape of testosterone replacement therapy continues to evolve as research expands our understanding of both benefits and risks. Therefore, practitioners must stay informed about emerging evidence while applying these updated guidelines with clinical judgment. Ultimately, successful testosterone replacement therapy requires balancing scientific evidence with patient-specific factors to optimize outcomes while minimizing potential harms.
Key Takeaways
Recent updates to testosterone replacement therapy guidelines reflect evolving evidence and challenge traditional diagnostic and treatment approaches, emphasizing individualized care and comprehensive safety monitoring.
- Diagnostic thresholds vary significantly: Professional organizations now recommend different testosterone cutoffs (264-300 ng/dL), requiring two morning samples and consideration of free testosterone in borderline cases.
- Treatment prioritizes symptom relief over levels: Goals focus on improving patient symptoms while targeting mid-normal testosterone ranges, with shared decision-making essential for borderline cases.
- Lifestyle modifications come first: Weight loss and exercise serve as first-line interventions for functional hypogonadism before considering testosterone therapy initiation.
- Formulation choice impacts outcomes: Transdermal gels provide stable levels with fewer cardiovascular risks compared to injections, while intranasal options preserve fertility.
- Safety monitoring remains critical: Regular hematocrit, PSA, and cardiovascular assessments are mandatory, with specific thresholds (50-54% hematocrit) guiding treatment decisions.
- Cardiovascular safety concerns reduced: Recent TRAVERSE trial evidence led FDA to remove cardiovascular warnings, though vigilance for pulmonary embolism and atrial fibrillation continues.
The key shift represents moving from one-size-fits-all protocols to personalized medicine approaches that balance individual patient factors, symptom improvement goals, and comprehensive risk assessment throughout treatment.
Frequently Asked Questions:
FAQs
Q1. What are the current diagnostic thresholds for testosterone deficiency? Recent guidelines suggest varying thresholds, with the American Urological Association using 300 ng/dL and the Endocrine Society recommending 264 ng/dL. Some organizations propose age-adjusted ranges, starting higher for younger men and decreasing with age.
Q2. How has the FDA’s stance on testosterone therapy’s cardiovascular risks changed? Based on recent evidence from the TRAVERSE trial, the FDA has removed language from the Boxed Warning related to adverse cardiovascular outcomes. However, vigilance is still recommended for specific cardiovascular events.
Q3. What are the primary goals of testosterone replacement therapy? The main objectives are to improve symptoms and achieve mid-normal testosterone levels. Treatment success is evaluated based on symptom relief and reaching targeted testosterone concentrations, typically within 3-5 ng/mL.
Q4. Are there testosterone formulations that can preserve fertility? Yes, intranasal testosterone (Natesto) offers a unique option for men concerned about fertility preservation. Clinical trials have shown that it can increase testosterone levels while largely maintaining semen parameters and sperm counts.
Q5. How often should hematocrit be monitored during testosterone therapy? Hematocrit should be measured before starting therapy and then at 3, 6, and 12 months. Guidelines vary on the threshold for concern, with American practices using 50% and European guidelines using 54% as the upper limit.
References:
[1] – https://www.nejm.org/doi/full/10.1056/NEJMoa2215025
[2] – https://pmc.ncbi.nlm.nih.gov/articles/PMC7520594/
[3] – https://pmc.ncbi.nlm.nih.gov/articles/PMC2948422/
[4] – https://pmc.ncbi.nlm.nih.gov/articles/PMC5647167/
[5] – https://en.wikipedia.org/wiki/Testosterone_undecanoate
[6] – https://pubmed.ncbi.nlm.nih.gov/34181890/
[7] – https://pmc.ncbi.nlm.nih.gov/articles/PMC1472884/
[8] – https://pmc.ncbi.nlm.nih.gov/articles/PMC9552794/
[9] – https://pmc.ncbi.nlm.nih.gov/articles/PMC6463248/
[10] – https://pubmed.ncbi.nlm.nih.gov/36253229/
[11] – https://www.auanet.org/guidelines-and-quality/guidelines/testosterone-deficiency-guideline
[12] – https://www.tandfonline.com/doi/abs/10.1080/17446651.2023.2296022
[13] – https://pmc.ncbi.nlm.nih.gov/articles/PMC7308235/
[14] – https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2293080
[15] – https://pubmed.ncbi.nlm.nih.gov/18042506/
[16] – https://www.fertstert.org/article/S0015-0282(19)32051-5/fulltext
[17] – https://pmc.ncbi.nlm.nih.gov/articles/PMC7108987/
[18] – https://pubmed.ncbi.nlm.nih.gov/32294396/
[19] – https://academic.oup.com/smr/article/13/1/33/7759906
[20] – https://pmc.ncbi.nlm.nih.gov/articles/PMC9835814/
[21] – https://transcare.ucsf.edu/testosterone-long-acting-pellets-testopel
[22] – https://pmc.ncbi.nlm.nih.gov/articles/PMC9006970/
[23] – https://pmc.ncbi.nlm.nih.gov/articles/PMC5182226/
[24] – https://www.aafp.org/pubs/afp/issues/2017/1001/p441.html
[25] – https://pmc.ncbi.nlm.nih.gov/articles/PMC6823728/
[26] – https://www.fda.gov/drugs/drug-safety-and-availability/fda-issues-class-wide-labeling-changes-testosterone-products
[27] – https://www.acc.org/Latest-in-Cardiology/Journal-Scans/2023/06/20/14/42/cardiovascular-safety-of-testosterone

