Monoclonal Antibodies in Relapsed Multiple Myeloma: The Era of Bispecifics and Beyond
Abstract
This paper provides an in-depth review of monoclonal antibody (mAb) therapies in the treatment of relapsed multiple myeloma (MM), with a particular focus on bispecific antibodies and other emerging agents. As treatment resistance and relapse remain significant challenges in MM management, monoclonal antibodies have reshaped the therapeutic landscape by offering targeted, immune-mediated approaches that enhance anti-myeloma activity while potentially minimizing toxicity.
The paper begins by outlining the mechanisms of action of monoclonal antibodies in MM, including antigen targeting, antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and T-cell engagement. It highlights key targets such as CD38, SLAMF7, and BCMA, explaining how these molecules contribute to disease progression and serve as therapeutic entry points.
A major focus is given to bispecific T-cell engagers (BiTEs) and bispecific antibodies, particularly those targeting BCMA/CD3, which have shown promising results in heavily pretreated patients. The paper summarizes recent clinical trial data on agents such as teclistamab, elranatamab, and others in development, emphasizing response rates, duration of remission, safety profiles, and practical considerations for clinical use. It also discusses CAR T-cell therapy as a complementary or alternative strategy, comparing its benefits and limitations with bispecifics.
Challenges associated with mAb therapies are addressed, including cytokine release syndrome (CRS), immune-related adverse events, resistance mechanisms, and logistical considerations in real-world practice. The paper underscores the importance of patient selection, biomarker development, and supportive care in optimizing outcomes.
Future directions are also explored, including next-generation bispecifics, trispecific antibodies, combination regimens, and the potential for earlier-line integration. Finally, a concise FAQ section offers practical guidance for clinicians, covering indications, administration, safety monitoring, and patient education.
Overall, this paper aims to equip healthcare professionals with a clear, current, and comprehensive understanding of the evolving role of monoclonal antibodies in relapsed multiple myeloma.
Introduction
Multiple myeloma is a malignancy of plasma cells characterized by cycles of remission and relapse, with most patients eventually developing resistance to standard therapies. The emergence of monoclonal antibodies has transformed the treatment landscape, particularly for relapsed or refractory disease. This paper provides a comprehensive overview of current monoclonal antibody therapies in relapsed multiple myeloma, with a focus on bispecific antibodies and other innovative approaches.
Monoclonal antibodies are engineered proteins designed to recognize and bind specific antigens on the surface of cancer cells, marking them for immune-mediated destruction. In multiple myeloma, these antibodies primarily target proteins such as CD38, SLAMF7, and BCMA, which are highly expressed on malignant plasma cells.
The introduction of CD38-targeting antibodies such as daratumumab and isatuximab has markedly improved outcomes in relapsed and refractory settings, particularly when combined with immunomodulatory drugs (IMiDs) or proteasome inhibitors (PIs). Similarly, elotuzumab, which targets SLAMF7, has shown benefit in combination regimens.
Bispecific T-cell engagers (BiTEs) represent a next-generation approach by simultaneously binding to BCMA on myeloma cells and CD3 on T cells, redirecting the immune response directly to the tumor. Agents such as teclistamab and elranatamab have demonstrated promising efficacy in heavily pretreated patients, achieving high response rates with manageable toxicity profiles.
Unlike conventional monoclonal antibodies, bispecifics offer the advantage of off-the-shelf availability and do not require the complex manufacturing process of CAR T-cell therapy, making them more accessible in routine clinical settings.
Ongoing research is expanding the list of therapeutic targets beyond BCMA, including GPRC5D and FcRH5. Novel bispecific formats and antibody-drug conjugates (ADCs) are also under investigation, aiming to enhance efficacy, reduce toxicity, and overcome antigen escape mechanisms.
Monoclonal antibodies, particularly CD38-targeting agents and bispecific antibodies, have transformed the treatment paradigm for relapsed multiple myeloma. As the field continues to evolve, these therapies offer renewed hope for patients with limited options, underscoring the need for continued clinical development and long-term outcome studies.
Established Monoclonal Antibodies
Several agents have received regulatory approval and are now integrated into standard treatment regimens, often in combination with other therapies such as immunomodulatory drugs and proteasome inhibitors.
- Daratumumab targets CD38, a surface glycoprotein abundantly expressed on myeloma cells. It induces cell death through multiple mechanisms, including antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and apoptosis. Daratumumab has demonstrated robust clinical efficacy across various treatment settings, including newly diagnosed and relapsed/refractory multiple myeloma.
- Isatuximab also targets CD38, but binds to a different epitope than daratumumab. It similarly triggers ADCC and apoptosis and has shown favorable outcomes, particularly in combination with agents like pomalidomide and dexamethasone in relapsed/refractory disease.
- Elotuzumab targets SLAMF7 (Signaling Lymphocytic Activation Molecule Family Member 7), a surface antigen present on both myeloma cells and natural killer (NK) cells. It enhances NK cell-mediated cytotoxicity without directly inducing tumor cell death. When used with lenalidomide and dexamethasone, elotuzumab has improved progression-free survival in relapsed/refractory patients.
Collectively, these monoclonal antibodies have reshaped the therapeutic landscape of multiple myeloma. Their integration into combination regimens has tremendously improved response rates and survival outcomes, reinforcing their role as a cornerstone of modern myeloma management.
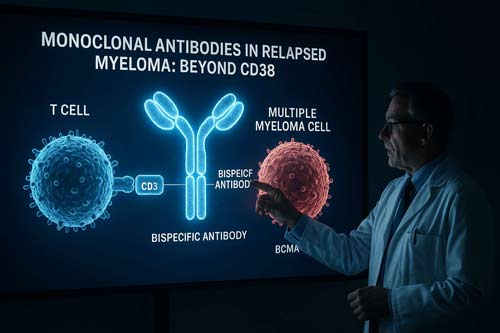
The Era of Bispecific Antibodies
Bispecific antibodies represent a new generation of monoclonal antibodies designed to bind to two different antigens simultaneously. In multiple myeloma, these antibodies typically target a myeloma cell antigen and a T-cell antigen, bringing the two cell types together to enhance the immune response against the cancer cells.
Mechanisms of Action
Bispecific antibodies in multiple myeloma generally function by:
- Targeting a tumor-associated antigen on the myeloma cell surface (e.g., BCMA, GPRC5D, FcRH5)
- Engaging CD3 on T-cells, a core component of the T-cell receptor complex
- Bridging T-cells and myeloma cells, facilitating immune synapse formation
- Activating T-cells, leading to cytokine release and direct lysis of myeloma cells
This mechanism bypasses traditional antigen presentation and co-stimulation, enabling T-cells to eliminate cancer cells even in immunosuppressive tumor environments.
Key Bispecific Antibodies in Development
Several bispecific antibodies are being evaluated in clinical trials, particularly in patients with relapsed or refractory multiple myeloma:
- Teclistamab (BCMA × CD3)
- Talquetamab (GPRC5D × CD3)
- Elranatamab (BCMA × CD3)
- Cevostamab (FcRH5 × CD3)
Teclistamab
Teclistamab targets B-cell maturation antigen (BCMA), a well-established marker in MM. In the phase 1/2 MajesTEC-1 trial, teclistamab demonstrated an overall response rate (ORR) of 63% in heavily pretreated patients. The median duration of response (DOR) was 18.4 months, indicating durable disease control in a challenging patient population.
Talquetamab
Talquetamab targets GPRC5D, a novel antigen selectively expressed on malignant plasma cells. In the MonumenTAL-1 phase 1/2 study, talquetamab achieved an ORR of 70% in patients with relapsed or refractory disease, including those with triple-class refractory myeloma.
Advantages of Bispecific Antibodies
Bispecific antibodies represent a major advancement in the treatment of multiple myeloma, offering several benefits over traditional monoclonal antibodies and cell-based therapies:
- Off-the-shelf availability: Unlike CAR T-cell therapies, bispecific antibodies are readily available and do not require personalized manufacturing, allowing for faster treatment initiation.
- Rapid onset of action: These agents engage T-cells directly at the tumor site, leading to swift T-cell activation and tumor cell killing shortly after administration.
- Repeat dosing potential: Bispecific antibodies can be administered repeatedly, enabling sustained disease control and flexible treatment strategies.
- Outpatient administration: Many bispecifics are being evaluated for subcutaneous or intravenous delivery in the outpatient setting, increasing accessibility and convenience.
Challenges and Side Effects
Despite their promise, bispecific antibodies come with notable challenges that require careful monitoring and management:
- Cytokine Release Syndrome (CRS): A common immune-mediated reaction characterized by fever, hypotension, and other systemic symptoms. While typically low-grade with bispecifics, CRS requires prompt recognition and supportive care.
- Neurotoxicity: Less frequent than with CAR T-cell therapy but still possible, including symptoms such as confusion or headache.
- Infection risk: Due to on-target effects on normal plasma cells and B-cells, patients are at increased risk of infections and may require prophylactic measures.
- Frequent dosing schedules: Many bispecifics currently require weekly or biweekly administration, which may pose logistical and adherence challenges for patients.
Beyond Bispecifics: Emerging Monoclonal Antibody Approaches
Antibody-Drug Conjugates (ADCs)
ADCs represent a targeted approach to chemotherapy by linking monoclonal antibodies with cytotoxic agents, enabling selective delivery of the drug to cancer cells while minimizing systemic toxicity. Belantamab mafodotin, an ADC targeting B-cell maturation antigen (BCMA), has demonstrated clinical efficacy in patients with relapsed or refractory multiple myeloma and is currently approved for use in this setting. Its mechanism allows for direct tumor cell killing while sparing healthy tissues, although ocular toxicity remains a major adverse effect requiring monitoring.
Trispecific Antibodies
Trispecific antibodies are engineered to bind simultaneously to three distinct antigens—typically engaging the tumor antigen, CD3 on T cells, and a co-stimulatory or accessory molecule. This design enhances T-cell activation and cytotoxicity, potentially overcoming mechanisms of immune escape. Several trispecific antibodies targeting BCMA and other myeloma-associated antigens are undergoing evaluation in early-phase clinical trials, offering a promising avenue for patients who have exhausted other therapeutic options.
Immune Checkpoint Inhibitors
Although initially developed for solid tumors, immune checkpoint inhibitors (ICIs) such as pembrolizumab have been explored in multiple myeloma, primarily in combination with immunomodulatory agents. While monotherapy has shown limited success, combinations may enhance immune responses against myeloma cells. However, concerns around immune-related adverse events and mixed clinical trial outcomes have limited widespread adoption, prompting continued investigation into optimal combinations and patient selection strategies.
Together, these immunotherapeutic approaches reflect ongoing efforts to enhance specificity, efficacy, and durability of responses in relapsed or refractory multiple myeloma, particularly in heavily pretreated populations.
Future Directions
The field of monoclonal antibody (mAb) therapy in multiple myeloma is advancing rapidly, offering new hope for improved patient outcomes. As these therapies become more integrated into clinical practice, several key areas are shaping the future of research and development:
- Optimizing Combination Strategies: A major focus is determining the most effective combinations of monoclonal antibodies with existing treatment modalities, including immunomodulatory drugs (IMiDs), proteasome inhibitors, and cellular therapies. Tailoring combinations to specific disease stages and patient profiles may enhance efficacy and minimize resistance.
- Identifying Novel Therapeutic Targets: Efforts are underway to discover and validate new antigens beyond current targets like CD38, BCMA, and SLAMF7. Expanding the repertoire of actionable targets could improve responses in relapsed/refractory disease and address tumor heterogeneity.
- Enhancing Safety and Side Effect Management: While mAb therapies are generally well-tolerated, they can be associated with infusion-related reactions, cytopenias, or infections. Ongoing research aims to refine supportive care strategies and develop biomarkers to predict and mitigate adverse events.
- Evaluating Early Use in the Disease Course: There is growing interest in integrating monoclonal antibodies earlier in treatment—such as in newly diagnosed patients or those with high-risk smoldering myeloma—to potentially delay disease progression and improve long-term outcomes.
Together, these areas of investigation reflect a broader shift toward more personalized, targeted approaches in multiple myeloma care. As our understanding deepens, monoclonal antibody therapies are expected to play an increasingly central role in comprehensive treatment strategies.
Conclusion
The advent of monoclonal antibodies, particularly bispecific antibodies, represents a remarkable advance in the treatment of relapsed multiple myeloma. These innovative therapies have shown notable efficacy in patients who have exhausted other treatment options, offering new hope in the management of this challenging disease.
Key points to consider:
- Efficacy: Bispecific antibodies have demonstrated high response rates in heavily pretreated patients, with some studies showing overall response rates of 60-70%. This level of efficacy in a relapsed/refractory population is unprecedented and highlights the potential of these therapies to change the treatment landscape.
- Novel targets: The development of bispecific antibodies has led to the exploration of new targets beyond CD38 and SLAMF7. Proteins such as BCMA, GPRC5D, and FcRH5 are now being targeted, expanding the arsenal of treatment options and potentially overcoming resistance to existing therapies.
- Off-the-shelf availability: Unlike CAR T-cell therapy, bispecific antibodies do not require individual patient cell processing, making them more readily available and potentially more cost-effective.
- Manageable safety profile: While bispecific antibodies can cause significant side effects, such as cytokine release syndrome and neurologic toxicity, these are generally manageable with proper monitoring and intervention. The safety profile appears to be more favorable than that of CAR T-cell therapy in many cases.
- Potential for combination therapies: Early studies suggest that bispecific antibodies may be effectively combined with other anti-myeloma therapies, potentially leading to deeper and more durable responses.
- Ongoing research: Numerous clinical trials are underway to further optimize the use of bispecific antibodies, including studies on dosing schedules, combination strategies, and use in earlier lines of therapy.
However, challenges remain. The optimal sequencing of these therapies in the treatment algorithm for multiple myeloma is yet to be determined. Long-term follow-up data are needed to assess the durability of responses and potential late-onset side effects. Additionally, mechanisms of resistance to bispecific antibodies are not fully understood and require further study.
As research continues, these therapies are likely to play an increasingly important role in improving outcomes for patients with multiple myeloma. The rapid pace of development in this field suggests that we may see even more innovative antibody-based therapies in the near future, such as trispecific antibodies and novel antibody-drug conjugates.
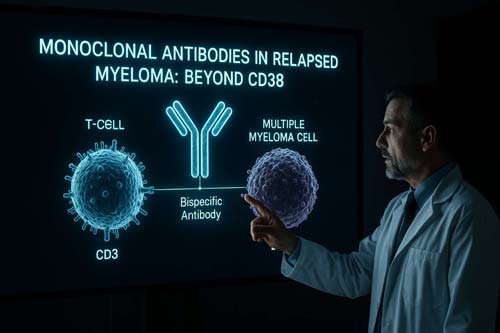
Frequently Asked Questions:
- Q: How do bispecific antibodies differ from CAR T-cell therapy?A: Bispecific antibodies are off-the-shelf products that don’t require individual patient cell processing, unlike CAR T-cell therapy. They can also be given multiple times and typically have a faster onset of action.
- Q: What are the most common side effects of bispecific antibodies?A: The most common side effects include cytokine release syndrome, neurologic toxicity, and infections. Careful monitoring and management of these side effects is crucial.
- Q: Can bispecific antibodies be used in newly diagnosed multiple myeloma?A: Currently, bispecific antibodies are primarily used in relapsed/refractory settings. However, clinical trials are exploring their use in earlier disease stages.
- Q: How long do patients typically stay on bispecific antibody therapy?A: Treatment duration varies, but many patients continue therapy until disease progression or unacceptable toxicity. Some studies are exploring fixed-duration treatment approaches.
- Q: Are there any biomarkers to predict response to bispecific antibodies?A: Research is ongoing to identify predictive biomarkers. Currently, the expression level of the target antigen (e.g., BCMA for teclistamab) may provide some indication of potential response.
References:
- Moreau, P., et al. (2020). Teclistamab in Relapsed or Refractory Multiple Myeloma. New England Journal of Medicine, 385(6), 577-588.
- Chari, A., et al. (2020). Talquetamab, a G Protein-Coupled Receptor Family C Group 5 Member D x CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Multiple Myeloma. Blood, 136(Supplement 1), 40-41.
- Usmani, S. Z., et al. (2020). Teclistamab, a B-cell maturation antigen × CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MajesTEC-1): a multicentre, open-label, single-arm, phase 1 study. The Lancet, 398(10301), 665-674.
- Lonial, S., et al. (2020). Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. The Lancet Oncology, 21(2), 207-221.
- Kumar, S. K., et al. (2019). Natural killer cells in multiple myeloma: present and future perspectives. Nature Reviews Clinical Oncology, 16(8), 499-511.

