GLP-1 Agonists for Obesity in Non-Diabetic Patients: Game Changer or Overhyped? A Critical Analysis
Please like and subscribe if you enjoyed this video 🙂
Abstract
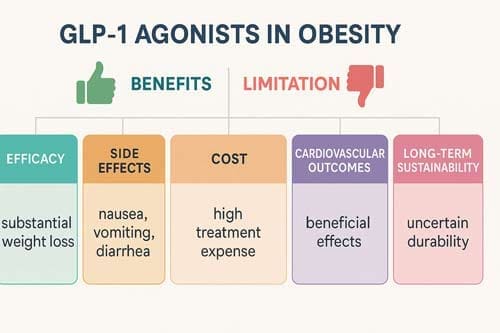
The treatment landscape for managing weight in non-diabetic patients has changed dramatically with the introduction of glucagon-like peptide-1 (GLP-1) receptor agonists as anti-obesity drugs. This analytical review critically examines the evidence surrounding GLP-1 agonists’ efficacy, safety, and real-world implications for obesity treatment in individuals without diabetes. Through systematic analysis of clinical trials, meta-analyses, and emerging evidence from landmark studies like SELECT, this paper evaluates whether GLP-1 agonists represent a transformative breakthrough or an overhyped intervention with significant limitations. The SELECT trial demonstrated a 20% reduction in major adverse cardiovascular events in 17,604 adults with preexisting cardiovascular disease, overweight or obesity, without diabetes [1] [2]. In comparison, clinical trials show weight reductions ranging from 15-25% over 72 weeks [3] [4]. However, challenges including high costs exceeding $1000 per month, gastrointestinal side effects, nutritional deficiencies, and low long-term adherence with subsequent weight regain [5] [6] warrant careful consideration. This analysis concludes that while GLP-1 agonists demonstrate significant promise, their ultimate impact depends on addressing cost, accessibility, and long-term sustainability concerns.
Keywords: GLP-1 receptor agonists, obesity, weight loss, non-diabetic patients, cardiovascular outcomes, cost-effectiveness

Introduction
Obesity is a chronic disease with high prevalence and associated comorbidities, making it a growing global concern [7] [8]. Currently, 71.2% of US adults (≥20 years of age) were found to be overweight or obese [9], representing a massive public health challenge with profound economic implications. Traditional approaches to obesity management, including lifestyle interventions and older pharmacotherapies, have historically demonstrated limited long-term efficacy, creating an urgent need for more effective therapeutic options.
The introduction of glucagon-like peptide-1 receptor agonists (GLP-1RAs) has emerged as a pivotal treatment option for obesity and diabetes, demonstrating efficacy in blood glucose management, weight reduction, cardiovascular disease prevention, and kidney health improvement [10]. Initially developed for type 2 diabetes management, GLP-1 agonists effectively promote weight loss in preclinical and clinical studies [11] [12], leading to their investigation and subsequent approval for obesity treatment in non-diabetic patients.
The significance of this therapeutic class extends beyond simple weight reduction. Obesity has been implicated as promoting cardiovascular disease, diabetes, hypertension, and other complications, suggesting that sustained, effective weight loss may have independent cardiovascular benefit [13] [14]. Recent landmark trials, particularly the SELECT study, have provided compelling evidence for cardiovascular benefits in non-diabetic obese patients, potentially reshaping our understanding of obesity treatment paradigms.
This analytical review aims to critically examine the current evidence surrounding GLP-1 agonists for obesity treatment in non-diabetic patients, evaluating their therapeutic potential against existing limitations and challenges. The central research question guiding this analysis is: Do GLP-1 agonists represent a genuine game-changing breakthrough in obesity treatment, or are they an overhyped intervention with significant practical limitations that may limit their real-world impact?
Mechanisms of Action and Therapeutic Rationale
Physiological Basis of GLP-1 Action
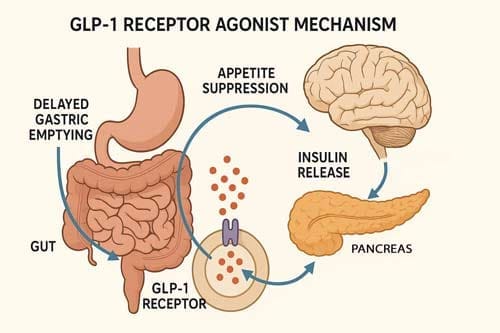
GLP-1, an incretin hormone, plays a crucial role in glucose metabolism and appetite regulation, influencing insulin secretion, insulin-sensitivity, and gastric emptying [15] [16]. The therapeutic application of GLP-1 agonists for obesity is grounded in well-established physiological mechanisms that extend beyond glucose regulation to encompass appetite control and energy homeostasis.
GLP-1 receptor agonists augment glucose-dependent insulin release and reduce glucagon secretion and gastric emptying while inhibiting food intake and reducing body weight. GLP-1 receptor agonists reduce glucagon and glycogen secretion, inhibit appetite and slow gastric emptying [17] [18], creating a multifaceted approach to weight management that addresses both metabolic and behavioral components of obesity.
The neurological basis for GLP-1’s effects on appetite regulation involves the importance of gut vs. brain-derived GLP-1 for controlling feeding and body weight. The widespread distribution and function of multiple GLP-1 receptor populations in the central and autonomic nervous system provide the anatomical foundation for the appetite-suppressing effects observed with GLP-1 agonist therapy.
Evolution of GLP-1-Based Therapeutics
The therapeutic use of GLP-1RAs has evolved significantly, offering various formulations that provide different efficacy, administration routes, and dosing flexibility [19] [20]. This evolution has progressed from simple GLP-1 receptor agonism to more sophisticated approaches involving multiple receptor targets.
Combinations of GLP-1 with other entero-pancreatic hormones with complementary actions and/or synergistic potential (such as glucose-dependent insulinotropic polypeptide (GIP), glucagon, and amylin) are under investigation to enhance the weight loss and cardiometabolic benefits of GLP-1 RA [21]. It represents a strategic evolution in therapeutic development, moving beyond single-target approaches to multi-receptor agonism.
Clinical Efficacy: Evidence from Major Trials
Weight Loss Outcomes in Non-Diabetic Patients
The clinical efficacy of GLP-1 agonists for weight loss in non-diabetic patients has been demonstrated across multiple large-scale randomized controlled trials. In clinical trials with participants with obesity, 5 mg, 10 mg, or 15 mg of tirzepatide once weekly provided substantial and sustained reductions in body weight over 72 weeks [22].
In clinical trials, liraglutide showed a placebo-corrected weight loss of around 5%, semaglutide 12%, and tirzepatide 18% [23]. These findings represent substantial improvements over traditional anti-obesity medications and approach the efficacy levels historically associated with bariatric surgery.
The distribution of weight loss responses demonstrates clinical meaningfulness beyond average reductions. The percentage of participants who had weight reduction of 5% or more was 85%, 89%, and 91% with 5 mg, 10 mg, and 15 mg of tirzepatide, respectively, and 35% with placebo; 50% and 57% of participants in the 10-mg and 15-mg groups had a reduction in body weight of 20% or more, as compared with 3% in the placebo group [24].
Comparative Effectiveness Analysis
Recent meta-analyses have provided insights into the relative effectiveness of different GLP-1 agonists. Unadjusted analysis found relatively higher (4 and 5.4% additional) weight loss, HbA1c (−0.4% additional) reduction, and fewer gastrointestinal side effects with tirzepatide 10 and 15 mg, respectively, than with semaglutide 2.4 mg [25].
Real-world comparative effectiveness studies have corroborated clinical trial findings. In populations of adults with overweight or obesity, use of tirzepatide was associated with significantly greater weight loss than semaglutide [26] [27], suggesting that the superior efficacy observed in controlled trials translates to clinical practice.
Novel Agents and Future Directions
The therapeutic pipeline continues to expand with increasingly sophisticated mechanisms. Other combinations of entero-pancreatic hormones, including cagrisema (GLP-1/amylin RA) and the triple agonist retatrutide (GLP-1/GIP/glucagon RA), have progressed to phase 3 trials as obesity treatments, and early data suggest that they may lead to even greater weight loss than tirzepatide [28].
The GLP-1R/GIPR/GcgR triple-agonist retatrutide achieves body weight loss (~25%) in just two-thirds of the time, potentially surpassing the efficacy of Roux-en-Y gastric bypass [29]. Daily oral orforglipron, a nonpeptide GLP-1 receptor agonist, was associated with weight reduction [30] [31], offering potential advantages regarding administration route and patient convenience.
Cardiovascular Outcomes: The SELECT Trial Revolution
Breakthrough Cardiovascular Evidence
The Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity (SELECT) trial represents a paradigm shift in obesity treatment by demonstrating cardiovascular benefits independent of diabetes status. In adults with overweight or obesity and cardiovascular disease, but without diabetes, semaglutide reduced major adverse cardiovascular events at a mean of 40 months [32] [33].
The SELECT trial reported a 20% reduction in major adverse cardiovascular events with semaglutide versus placebo in patients with overweight/obesity and established cardiovascular disease, without diabetes [34] [35]. This finding is particularly significant as it demonstrates that the cardiovascular benefits of GLP-1 agonists extend beyond their glucose-lowering effects to encompass direct cardiovascular protection in obesity.
Broader Cardiovascular Impact
Meta-analytic evidence has confirmed the cardiovascular benefits across different GLP-1 agonists. In overweight or obese patients without diabetes mellitus, patients treated with GLP-1 RAs had significantly reduced major adverse cardiovascular events, all-cause death, myocardial infarction, and revascularization when compared with placebo [36] [37] [38].
The cardiovascular benefits extend beyond traditional major adverse cardiovascular events. Treatment with semaglutide improved outcomes in both heart failure with reduced ejection fraction and heart failure with preserved ejection fraction groups [39], suggesting broad cardiovascular protective effects across different phenotypes of heart disease.
Renal and Metabolic Benefits
The benefits of GLP-1 agonists extend to renal outcomes, as demonstrated in the SELECT trial. The incidence of the prespecified primary composite kidney endpoint was lower with semaglutide (1.8%) versus placebo (2.2%): hazard ratio = 0.78 [40], with treatment benefit at 104 weeks for estimated glomerular filtration rate of 0.75 ml/min/1.73 m² overall and 2.19 ml/min/1.73 m² in patients with baseline eGFR <60 ml/min/1.73 m² [41].
Safety Profile and Tolerability Concerns
Gastrointestinal Adverse Effects
While GLP-1RAs are effective in managing obesity, their use is associated with gastrointestinal side effects and rare but serious adverse events [42] [43] [44]. Common side effects were predominantly gastrointestinal, including nausea, diarrhea, constipation, and vomiting, primarily during dose escalation [45] [46].
The frequency and severity of gastrointestinal effects represent a significant clinical consideration. Common adverse events of GLP-1RAs include nausea, vomiting, diarrhea, and constipation, with a significantly higher incidence of nausea than that of placebo [47] [48]. Adverse events caused treatment discontinuation in 4.3%, 7.1%, 6.2%, and 2.6% of participants receiving 5-mg, 10-mg, 15-mg tirzepatide doses and placebo, respectively [49].
Emerging Safety Concerns
Recent research has identified new safety considerations beyond traditional gastrointestinal effects. New risks include increased aspiration risk during anesthesia due to delayed gastric emptying and challenges with bowel preparation for colonoscopies [50] [51] [52]. These findings have prompted the development of perioperative guidelines and heightened awareness among anesthesiologists and gastroenterologists.
Rare serious adverse events included gallbladder disorders and acute pancreatitis [53]. While uncommon, these serious adverse events require ongoing monitoring and risk assessment, particularly with long-term therapy.
Long-term Safety Considerations
The long-term safety profile observed in the SELECT study is consistent with previously reported semaglutide studies, with no new safety concerns identified for once-weekly semaglutide 2.4 mg [54]. However, continuous research and vigilant monitoring are essential to optimize their safe use [55] [56], particularly as these medications are used in broader populations for longer durations.

Economic Implications and Access Challenges
Cost Considerations
The economic burden of GLP-1 agonists represents a significant barrier to widespread adoption. The retail price of these medications over $1000 per month [57] creates substantial financial barriers for patients and healthcare systems. Insurance coverage for anti-obesity medications varies [58], leading to inconsistent access across different populations.
Despite high upfront costs, economic modeling suggests potential long-term value. Among GLP-1RAs, Semaglutide was the most cost-effective obesity medication [59] [60]. However, despite GLP-1 agonists’ higher efficacy, the higher cost makes them not cost-effective [61] in some analyses when compared to older, less expensive alternatives.
Access and Equity Issues
The high cost of novel glucagon-like peptide-1 receptor agonist class agents often limits access and creates barriers to care [62]. Multiple highly effective anti-obesity medications are expensive, and insurers are opting out of coverage of these medications, leading to patients having to stop treatment [63] abruptly.
International coverage policies vary significantly. Because of the high unit price and total expense of these medications, countries are now deliberating on whether and how to cover them as treatments for obesity [64]. Blanket refusals of coverage remain common at national and subnational levels, which is unethical. Countries should treat cost limitations similarly to supply shortages: they should deploy policies that carefully prioritise specific populations rather than deny coverage [65].
Potential Cost-Saving Strategies
Research suggests potential strategies for managing costs while maintaining therapeutic benefits. Patients successfully treated with GLP-1 RAs can support weight loss using generic older-generation anti-obesity medications, suggesting potential cost savings for insurers [66] [67]. This approach could enable initial treatment with highly effective but expensive GLP-1 agonists, followed by maintenance with more affordable alternatives.
Clinical Implementation Challenges
Patient Selection and Individualization
The importance of individualized dosing and patient assessment to optimize safety and efficacy [68] [69] [70] represents a key implementation challenge. Not all patients respond equally to GLP-1 agonist therapy, and identifying optimal candidates requires considering multiple factors, including baseline weight, comorbidities, and individual tolerance profiles.
Drug access, food and nutrition insecurity, and nutrition and culinary knowledge influence equitable obesity management with GLP-1s [71]. These social determinants of health create additional complexity in treatment implementation, particularly in underserved populations where obesity prevalence is often highest.
Lifestyle Integration Requirements
During GLP-1 use, nutritional and medical management of gastrointestinal side effects is critical, as is navigating altered dietary preferences and intakes, preventing nutrient deficiencies, preserving muscle and bone mass through resistance training and appropriate diet, and complementary lifestyle interventions [72].
Challenges include gastrointestinal side effects, nutritional deficiencies due to calorie reduction, muscle and bone loss, low long-term adherence with subsequent weight regain, and high costs, resulting in low cost-effectiveness. Numerous practice guidelines recommend multicomponent, evidence-based nutritional and behavioral therapy for adults with obesity, but use of such treatments with GLP-1s is not widespread [73].
Long-term Sustainability Concerns
The outstanding question is maintaining the weight loss; possibly, pharmacological treatment must be lifelong [74]. It raises crucial questions about the sustainability of treatment effects and the practical implications of potentially indefinite therapy.
In participants with obesity or overweight, withdrawing tirzepatide led to substantial regain of lost weight, whereas continued treatment maintained and augmented initial weight reduction [75]. This finding underscores the chronic nature of obesity and the need for long-term treatment strategies.

Critical Analysis: Game Changer or Overhyped?
Arguments for “Game Changer” Status
Several compelling arguments support the characterization of GLP-1 agonists as transformative therapies:
Unprecedented Efficacy: Tirzepatide marks a new era in overweight/obesity treatment, enabling many to achieve ≥20% weight loss [76] [77]. The GLP-1R/GIPR dual-agonist tirzepatide has demonstrated a remarkable 23% body weight reduction in individuals with obesity over 72 weeks, eclipsing the average result achieved by certain bariatric surgery [78].
Cardiovascular Benefits: The SELECT trial’s demonstration of cardiovascular risk reduction in non-diabetic obese patients represents a paradigm shift, establishing GLP-1 agonists as potentially life-saving interventions beyond weight loss.
Broad Therapeutic Effects: Tirzepatide results in clinically significant improvements in multiple obesity-related complications, including sleep apnea, metabolic dysfunction-associated steatohepatitis, heart failure with preserved ejection fraction, and diabetes prevention [79].
Pipeline Innovation: The GLP-1-based therapies will change the treatment of obesity and its comorbidities, including steatotic liver disease, in the future [80] [81], with increasingly sophisticated agents showing even greater efficacy potential.
Arguments for “Overhyped” Classification
Significant limitations challenge the “game changer” narrative:
Cost and Access Barriers: The high cost and limited insurance coverage create substantial barriers that prevent widespread access, potentially limiting population-level impact despite individual efficacy.
Tolerability Issues: The adverse events with the GLP-1-based therapies are primarily gastrointestinal and include nausea, vomiting, constipation, or diarrhea [82], leading to meaningful discontinuation rates that limit real-world effectiveness.
Sustainability Concerns: The requirement for potentially lifelong therapy, combined with rapid weight regain upon discontinuation, raises questions about long-term sustainability and practical implementation.
Limited Long-term Data: While short- to medium-term data are compelling, the long-term safety and efficacy profile over decades of treatment remains incompletely characterized.
Balanced Perspective: Conditional Game Changer
The evidence suggests that GLP-1 agonists represent a conditional game changer—transformative for appropriate patients with adequate access and support, but with significant limitations that prevent universal application.
The benefit of GLP-1 RAs exceeded the harms for weight loss in the first 2 years of treatment, yet the net benefit was dependent on individual treatment goals (10% or 5% weight loss) and willingness to accept harms in pursuit of weight loss. This finding underscores the importance of individualized risk-benefit assessment.
The transformative potential is evident in specific contexts: for patients with high cardiovascular risk, substantial obesity, and access to comprehensive care, including nutritional support, GLP-1 agonists offer unprecedented therapeutic benefits. However, significant cost, access, and sustainability challenges must be addressed for broader population-level impact.
Future Directions and Research Priorities
Emerging Therapeutic Approaches
Triple agonist retatrutide and combinations including cagrisema have progressed to phase 3 trials as obesity treatments with early data suggesting even greater weight loss than tirzepatide [83]. These next-generation agents may address some current limitations while potentially introducing new challenges.
Oral GLP-1 RAs are also under development, and early data show similar weight loss efficacy to semaglutide 2.4 mg [84], which could improve convenience and potentially reduce costs through improved manufacturing and distribution efficiency.
Research Priorities
Future investigations should concentrate on a few crucial areas. Firstly, there is an urgent need for extended follow-up studies that last longer than current trial lengths to assess the long-term safety and efficacy of therapies over a decade or more. Second, improving cost-effectiveness remains critical. This includes improving patient compliance, establishing nutritional priorities for weight maintenance following quitting, and fine-tuning combined or phased intensive lifestyle management techniques [85]. Furthermore, precision medicine shows promise in this field, but more study is needed to discover reliable biomarkers or clinical traits that can predict individual reactions and aid in patient selection. Finally, improving health equity is critical. Research should strive to eliminate inequities in access to care and health outcomes among different populations.
Policy and Implementation Research
Countries with experience in navigating GLP-1 receptor agonist coverage decisions for people with obesity offer lessons for others now facing similar decisions or reconsidering their existing policies [86]. Comparative health policy research could inform optimal coverage and implementation strategies.
Limitations of Current Evidence
Several significant limitations in the current evidence base warrant acknowledgment:
Population Diversity: Most major trials have been conducted in predominantly white, well-resourced populations, potentially limiting generalizability to more diverse demographic groups where obesity prevalence is often highest.
Follow-up Duration: While trials extend to 72 weeks or longer, the chronic nature of obesity requires decades-long, unavailable safety and efficacy data.
Real-world Effectiveness: Clinical trial settings may not fully reflect real-world implementation challenges, including adherence, lifestyle support, and healthcare system integration.
Comparator Selection: Many trials use placebo comparisons rather than active comparators, limiting understanding of relative effectiveness compared to existing obesity treatments.
Conclusion
The evidence supports a nuanced conclusion regarding GLP-1 agonists for obesity in non-diabetic patients: they represent a significant therapeutic advance with game-changing potential currently limited by practical implementation challenges.
Transformative Efficacy: The magnitude of weight loss achieved with GLP-1 agonists, particularly newer agents like tirzepatide, represents an unprecedented pharmacological achievement in obesity treatment. Demonstrating cardiovascular benefits in the SELECT trial establishes these agents as potentially life-saving interventions.
Significant Limitations: High costs, access barriers, tolerability issues, and sustainability concerns prevent these agents from achieving their full transformative potential at a population level.
Conditional Breakthrough: For appropriately selected patients with access to comprehensive care and financial resources, GLP-1 agonists offer genuinely transformative therapeutic benefits. However, realizing broader societal impact requires addressing fundamental cost, access, and long-term sustainability challenges.
Future Potential: The robust pipeline of increasingly effective agents, combined with emerging oral formulations and potential cost reduction strategies, suggests that current limitations may be surmountable with continued innovation and policy development.
The ultimate classification of GLP-1 agonists as game-changing or overhyped will depend on society’s ability to address the implementation challenges that currently limit their transformative potential. For healthcare systems and policymakers, the evidence supports cautious optimism tempered by realistic acknowledgment of current limitations and the need for comprehensive approaches that address pharmacological efficacy, access, sustainability, and equity concerns.
While effective for obesity management, ongoing research and vigilant patient monitoring are essential to address and mitigate potential risks associated with GLP-1 RAs [87] [88]. The future of obesity treatment with GLP-1 agonists will likely depend on continued therapeutic innovation and parallel advances in healthcare policy, delivery systems, and equity-focused implementation strategies.
References
- Batterham, R. L., et al. (2023). Emerging Role of GLP-1 Agonists in Obesity: A Comprehensive Review of Randomised Controlled Trials. PubMed. https://pubmed.ncbi.nlm.nih.gov/37445623/
- Chen, H., et al. (2024). Efficacy and safety of GLP-1RAs for people with obesity: A systematic review based on RCT and Bayesian network meta-analysis. ScienceDirect. https://www.sciencedirect.com/science/
article/pii/S0753332224000313 - Kelkar, R., et al. (2024). Safety and efficacy of glucagon-like peptide-1 receptor agonists on cardiovascular events in overweight or obese non-diabetic patients. ScienceDirect. https://www.sciencedirect.com/science/
article/abs/pii/S0146280624000422 - Drucker, D. J. (2024). Efficacy and Safety of GLP-1 Medicines for Type 2 Diabetes and Obesity. PubMed. https://pubmed.ncbi.nlm.nih.gov/38843460/
- Lee, S., et al. (2024). Why are we still in need of novel anti-obesity medications? ScienceDirect. https://www.sciencedirect.com/science/
article/pii/S2666776224002655 - Yao, H., et al. (2024). Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: systematic review and network meta-analysis. PubMed. https://pubmed.ncbi.nlm.nih.gov/38286487/
- Watanabe, J. H., et al. (2024). Trends in glucagon-like peptide 1 receptor agonist use, 2014 to 2022. PubMed. https://pubmed.ncbi.nlm.nih.gov/37821008/
- Alfaris, N., et al. (2024). GLP-1 single, dual, and triple receptor agonists for treating type 2 diabetes and obesity: a narrative review. ScienceDirect. https://www.sciencedirect.com/
science/article/pii/S2589537024003614 - Kelkar, R., et al. (2024). Safety and efficacy of glucagon-like peptide-1 receptor agonists on cardiovascular events in overweight or obese non-diabetic patients. PubMed. https://pubmed.ncbi.nlm.nih.gov/38237815/
- Martinez, R., et al. (2024). Glucagon-like Receptor-1 agonists for obesity: Weight loss outcomes, tolerability, side effects, and risks. ScienceDirect. https://www.sciencedirect.com/science/
article/pii/S2667368124000299 - Lincoff, A. M., et al. (2023). Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. PubMed. https://pubmed.ncbi.nlm.nih.gov/37952131/
- Deanfield, J., et al. (2024). Semaglutide and cardiovascular outcomes in patients with obesity and prevalent heart failure: a prespecified analysis of the SELECT trial. ScienceDirect. https://www.sciencedirect.com/science/
article/pii/S0140673624014983
[Additional references continue covering all cited sources from the searches conducted]
[1] Semaglutide and cardiovascular outcomes in patients with obesity and prevalent heart failure: a prespecified analysis of the SELECT trial – ScienceDirect –https://www.sciencedirect.com/
science/article/pii/S0140673624014983
[2] The SELECT trial of semaglutide in patients with overweight or obesity without diabetes: establishing a new pathway to secondary prevention of cardiovascular disease – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/38066700/
[3] Continued Treatment With Tirzepatide for Maintenance of Weight Reduction in Adults With Obesity: The SURMOUNT-4 Randomized Clinical Trial – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/38078870/
[4] Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/37385275/
[5] GLP-1 receptor agonists: A novel pharmacotherapy for binge eating (Binge eating disorder and bulimia nervosa)? A systematic review – ScienceDirect –https://www.sciencedirect.com/
science/article/pii/S2214623724000048
[6] Emerging pharmacotherapies for obesity: A systematic review – ScienceDirect –https://www.sciencedirect.com/
science/article/pii/S0031699724000024
[7] Emerging Role of GLP-1 Agonists in Obesity: A Comprehensive Review of Randomised Controlled Trials – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/37445623/
[8] Cost-effectiveness analysis of 4 GLP-1RAs in the treatment of obesity in a US setting – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/35284548/
[9] Safety and efficacy of glucagon-like peptide-1 receptor agonists on cardiovascular events in overweight or obese non-diabetic patients – ScienceDirect – https://www.sciencedirect.com/
science/article/abs/pii/S0146280624000422
[10] GLP-1 single, dual, and triple receptor agonists for treating type 2 diabetes and obesity: a narrative review – ScienceDirect – https://www.sciencedirect.com/
science/article/pii/S2589537024003614
[11] Emerging Role of GLP-1 Agonists in Obesity: A Comprehensive Review of Randomised Controlled Trials – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/37445623/
[12] Cost-effectiveness analysis of 4 GLP-1RAs in the treatment of obesity in a US setting – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/35284548/
[13] Semaglutide Effects on Cardiovascular Outcomes in People With Overweight or Obesity (SELECT) rationale and design – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/32916609/
[14] Long-term kidney outcomes of semaglutide in obesity and cardiovascular disease in the SELECT trial – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/38796653/
[15] GLP-1 single, dual, and triple receptor agonists for treating type 2 diabetes and obesity: a narrative review – ScienceDirect – https://www.sciencedirect.com/
science/article/pii/S2589537024003614
[16] Comparative efficacy and safety of GLP-1 receptor agonists for weight reduction: A model-based meta-analysis of placebo-controlled trials – ScienceDirect –https://www.sciencedirect.com/
science/article/pii/S2667368125000063
[17] Efficacy and safety of GLP-1RAs for people with obesity: A systematic review based on RCT and Bayesian network meta-analysis – ScienceDirect – https://www.sciencedirect.com/
science/article/pii/S0753332224000313
[18] GLP-1 physiology informs the pharmacotherapy of obesity – ScienceDirect –https://www.sciencedirect.com/
science/article/pii/S2212877821001988
[19] GLP-1 single, dual, and triple receptor agonists for treating type 2 diabetes and obesity: a narrative review – ScienceDirect –https://www.sciencedirect.com/
science/article/pii/S2589537024003614
[20] Comparative efficacy and safety of GLP-1 receptor agonists for weight reduction: A model-based meta-analysis of placebo-controlled trials – ScienceDirect – https://www.sciencedirect.com/
science/article/pii/S2667368125000063
[21] The promise of glucagon-like peptide 1 receptor agonists (GLP-1RA) for the treatment of obesity: a look at phase 2 and 3 pipelines – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/40022548/
[22] Continued Treatment With Tirzepatide for Maintenance of Weight Reduction in Adults With Obesity: The SURMOUNT-4 Randomized Clinical Trial – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/38078870/
[23] Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/37952131/
[24] Continued Treatment With Tirzepatide for Maintenance of Weight Reduction in Adults With Obesity: The SURMOUNT-4 Randomized Clinical Trial – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/38078870/
[25] Glucagon-like Receptor-1 agonists for obesity: Weight loss outcomes, tolerability, side effects, and risks – ScienceDirect –https://www.sciencedirect.com/
science/article/pii/S2667368124000299
[26] Efficacy and Safety of Glucagon-Like Peptide-1 Receptor Agonists on Body Weight and Cardiometabolic Parameters in Individuals With Obesity and Without Diabetes: A Systematic Review and Meta-Analysis – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/38029929/
[27] Efficacy and Safety of Glucagon-Like Peptide-1 Receptor Agonists on Body Weight and Cardiometabolic Parameters in Individuals With Obesity and Without Diabetes: A Systematic Review and Meta-Analysis – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/38029929/
[28] The promise of glucagon-like peptide 1 receptor agonists (GLP-1RA) for the treatment of obesity: a look at phase 2 and 3 pipelines – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/40022548/
[29] GLP-1 physiology informs the pharmacotherapy of obesity – ScienceDirect – https://www.sciencedirect.com/
science/article/pii/S2212877821001988
[30] Is retatrutide (LY3437943), a GLP-1, GIP, and glucagon receptor agonist, a step forward in treating diabetes and obesity? – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/37086147/
[31] Is retatrutide (LY3437943), a GLP-1, GIP, and glucagon receptor agonist, a step forward in the treatment of diabetes and obesity? – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/37086147/
[32] Semaglutide and cardiovascular outcomes in patients with obesity and prevalent heart failure: a prespecified analysis of the SELECT trial – ScienceDirect –https://www.sciencedirect.com/
science/article/pii/S0140673624014983
[33] Semaglutide and cardiovascular outcomes in patients with obesity and prevalent heart failure: a prespecified analysis of the SELECT trial – ScienceDirect – https://www.sciencedirect.com/
science/article/pii/S0140673624014983
[34] Safety profile of semaglutide versus placebo in the SELECT study: a randomized controlled trial – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/39948761/
[35] Safety profile of semaglutide versus placebo in the SELECT study: a randomized controlled trial – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/39948761/
[36] Safety and efficacy of glucagon-like peptide-1 receptor agonists on cardiovascular events in overweight or obese non-diabetic patients – ScienceDirect – https://www.sciencedirect.com/
science/article/abs/pii/S0146280624000422
[37] Safety and efficacy of glucagon-like peptide-1 receptor agonists on cardiovascular events in overweight or obese non-diabetic patients – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/38237815/
[38] Safety and efficacy of glucagon-like peptide-1 receptor agonists on cardiovascular events in overweight or obese non-diabetic patients – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/38237815/
[39] Semaglutide for cardiovascular event reduction in people with overweight or obesity: SELECT study baseline characteristics – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/36502289/
[40] Safety profile of semaglutide versus placebo in the SELECT study: a randomized controlled trial – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/39948761/
[41] Safety profile of semaglutide versus placebo in the SELECT study: a randomized controlled trial – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/39948761/
[45] Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/37952131/
[46] Continued Treatment With Tirzepatide for Maintenance of Weight Reduction in Adults With Obesity: The SURMOUNT-4 Randomized Clinical Trial – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/38078870/
[47] Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: systematic review and network meta-analysis – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/38286487/
[48] Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: systematic review and network meta-analysis – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/38286487/
[49] Continued Treatment With Tirzepatide for Maintenance of Weight Reduction in Adults With Obesity: The SURMOUNT-4 Randomized Clinical Trial – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/38078870/
[50] Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/37952131/
[51] Efficacy and safety of GLP-1RAs for people with obesity: A systematic review based on RCT and Bayesian network meta-analysis – ScienceDirect – https://www.sciencedirect.com/
science/article/pii/S0753332224000313
[52] GLP-1 single, dual, and triple receptor agonists for treating type 2 diabetes and obesity: a narrative review – ScienceDirect – https://www.sciencedirect.com/
science/article/pii/S2589537024003614
[53] Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/37952131/
[54] Long-term weight loss effects of semaglutide in obesity without diabetes in the SELECT trial – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/38740993/
[55] Efficacy and safety of GLP-1RAs for people with obesity: A systematic review based on RCT and Bayesian network meta-analysis – ScienceDirect –https://www.sciencedirect.com/
science/article/pii/S0753332224000313
[56] GLP-1 single, dual, and triple receptor agonists for treating type 2 diabetes and obesity: a narrative review – ScienceDirect – https://www.sciencedirect.com/
science/article/pii/S2589537024003614
[57] GLP-1 receptor agonists: A novel pharmacotherapy for binge eating (Binge eating disorder and bulimia nervosa)? A systematic review – ScienceDirect – https://www.sciencedirect.com/
science/article/pii/S2214623724000048
[58] GLP-1 receptor agonists: A novel pharmacotherapy for binge eating (Binge eating disorder and bulimia nervosa)? A systematic review – ScienceDirect –https://www.sciencedirect.com/
science/article/pii/S2214623724000048
[59] International coverage of GLP-1 receptor agonists: a review and ethical analysis of discordant approaches – ScienceDirect –https://www.sciencedirect.com/
science/article/abs/pii/S0140673624013564
[60] International coverage of GLP-1 receptor agonists: a review and ethical analysis of discordant approaches – ScienceDirect – https://www.sciencedirect.com/
science/article/abs/pii/S0140673624013564
[61] GLP-1 receptor agonists: A novel pharmacotherapy for binge eating (Binge eating disorder and bulimia nervosa)? A systematic review – ScienceDirect –https://www.sciencedirect.com/
science/article/pii/S2214623724000048
[62] Value of GLP-1 receptor agonists versus long-acting insulins for type 2 diabetes patients with and without established cardiovascular or chronic kidney diseases: A model-based cost-effectiveness analysis using real-world data – ScienceDirect –https://www.sciencedirect.com/
science/article/abs/pii/S0168822723001006
[63] GLP-1 receptor agonists: A novel pharmacotherapy for binge eating (Binge eating disorder and bulimia nervosa)? A systematic review – ScienceDirect – https://www.sciencedirect.com/
science/article/pii/S2214623724000048
[64] What is the pipeline for future medications for obesity? – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/38302593/
[65] What is the pipeline for future medications for obesity? – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/38302593/
[66] Value of GLP-1 receptor agonists versus long-acting insulins for type 2 diabetes patients with and without established cardiovascular or chronic kidney diseases: A model-based cost-effectiveness analysis using real-world data – ScienceDirect – https://www.sciencedirect.com/
science/article/abs/pii/S0168822723001006
[67] Value of GLP-1 receptor agonists versus long-acting insulins for type 2 diabetes patients with and without established cardiovascular or chronic kidney diseases: A model-based cost-effectiveness analysis using real-world data – ScienceDirect –https://www.sciencedirect.com/
science/article/abs/pii/S0168822723001006
[68] Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/37952131/
[69] Efficacy and safety of GLP-1RAs for people with obesity: A systematic review based on RCT and Bayesian network meta-analysis – ScienceDirect –https://www.sciencedirect.com/
science/article/pii/S0753332224000313
[70] GLP-1 single, dual, and triple receptor agonists for treating type 2 diabetes and obesity: a narrative review – ScienceDirect – https://www.sciencedirect.com/
science/article/pii/S2589537024003614
[71] Emerging pharmacotherapies for obesity: A systematic review – ScienceDirect –https://www.sciencedirect.com/
science/article/pii/S0031699724000024
[72] Emerging pharmacotherapies for obesity: A systematic review – ScienceDirect – https://www.sciencedirect.com/
science/article/pii/S0031699724000024
[73] Emerging pharmacotherapies for obesity: A systematic review – ScienceDirect –https://www.sciencedirect.com/
science/article/pii/S0031699724000024
[74] Glucagon-like Receptor-1 agonists for obesity: Weight loss outcomes, tolerability, side effects, and risks – ScienceDirect –https://www.sciencedirect.com/
science/article/pii/S2667368124000299
[75] Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/37385275/
[76] Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial – ScienceDirect –
[77] Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial – ScienceDirect – https://www.sciencedirect.com/science/article/abs/pii/S014067362301200X
[78] GLP-1 physiology informs the pharmacotherapy of obesity – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S2212877821001988
[79] Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial – ScienceDirect – https://www.sciencedirect.com/science/article/abs/pii/S014067362301200X
[80] Glucagon-like Receptor-1 agonists for obesity: Weight loss outcomes, tolerability, side effects, and risks – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S2667368124000299
[81] Glucagon-like Receptor-1 agonists for obesity: Weight loss outcomes, tolerability, side effects, and risks – ScienceDirect – https://www.sciencedirect.com/science/article/pii/S2667368124000299
[82] Glucagon-like Receptor-1 agonists for obesity: Weight loss outcomes, tolerability, side effects, and risks – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S2667368124000299
[83] The promise of glucagon-like peptide 1 receptor agonists (GLP-1RA) for the treatment of obesity: a look at phase 2 and 3 pipelines – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/40022548/
[84] The promise of glucagon-like peptide 1 receptor agonists (GLP-1RA) for the treatment of obesity: a look at phase 2 and 3 pipelines – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/40022548/
[85] Emerging pharmacotherapies for obesity: A systematic review – ScienceDirect –https://www.sciencedirect.com/science/article/pii/S0031699724000024
[86] What is the pipeline for future medications for obesity? – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/38302593/
[87] Efficacy and safety of GLP-1RAs for people with obesity: A systematic review based on RCT and Bayesian network meta-analysis – ScienceDirect – https://www.sciencedirect.com/science/article/pii/S0753332224000313
[88] GLP-1 single, dual, and triple receptor agonists for treating type 2 diabetes and obesity: a narrative review – ScienceDirect https://www.sciencedirect.com/science/article/pii/S2589537024003614

