Anemia of Inflammation vs Iron Deficiency Anemia: New Diagnostic Tools and Therapies
Please like and subscribe if you enjoyed this video 🙂
Abstract
This paper provides a clear, up-to-date overview of how anemia of inflammation (AI) and iron deficiency anemia (IDA) differ, focusing on how to diagnose and treat them effectively. It highlights recent advances in diagnostic tools and therapies, helping healthcare professionals make informed decisions, especially when managing anemia in chronic disease.
Introduction
Anemia is a highly prevalent condition encountered across virtually all clinical specialties. It is defined by a reduction in healthy red blood cells or hemoglobin, impairs oxygen delivery throughout the body. Among its many forms, anemia of inflammation (AI) and iron deficiency anemia (IDA) are particularly common and often clinically indistinguishable at first glance. Despite overlapping presentations, these two types require distinct treatment strategies. This paper aims to clarify their differences, discuss diagnostic tools, and highlight current and emerging therapies relevant to modern clinical practice.
Understanding Anemia of Inflammation and Iron Deficiency Anemia
Anemia of inflammation, also known as anemia of chronic disease, typically arises in patients with long-term inflammatory conditions such as:
- Chronic Infections
Long-standing infections like tuberculosis, HIV, and osteomyelitis trigger ongoing immune responses. The inflammatory cytokines—especially IL-6—upregulate hepcidin production, limiting iron availability and suppressing erythropoiesis. Anemia may persist despite iron sufficiency and is often resistant to oral iron therapy alone. - Autoimmune and Rheumatologic Disorders
Conditions such as rheumatoid arthritis, systemic lupus erythematosus (SLE), and inflammatory vasculitis are commonly associated with AI. Chronic inflammation in these diseases promotes elevated hepcidin and disrupts iron homeostasis, often leading to normocytic or slightly microcytic anemia. - Malignancies
Solid tumors and hematologic cancers (e.g., lymphomas) can lead to AI through both direct marrow suppression and systemic inflammatory effects. Cancer-related anemia often involves a mixed pattern, with AI and nutritional deficiencies (e.g., IDA, B12 deficiency) coexisting. - Chronic Kidney Disease (CKD)
In CKD, anemia results from decreased erythropoietin production and chronic inflammation. Elevated hepcidin levels in this setting further reduce iron bioavailability, creating a functional iron deficiency. ESA resistance is common, and IV iron is often necessary for optimal management.
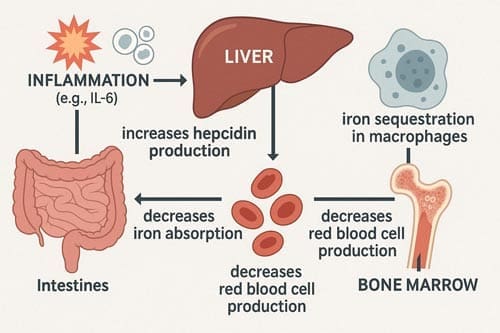
Inflammatory cytokines, particularly interleukin-6 (IL-6) stimulate the liver to produce hepcidin, which inhibits iron export from enterocytes and macrophages. As a result, iron becomes sequestered in storage sites and is unavailable for red blood cell production. Erythropoiesis is further suppressed by reduced responsiveness to erythropoietin. Despite adequate or even elevated iron stores, patients develop a functional iron deficiency.
Iron Deficiency Anemia (IDA)
Iron deficiency anemia (IDA) is caused by an absolute deficiency of iron, typically due to one or more of the following:
- Inadequate Dietary Intake
Common in populations with limited access to animal-based foods or in individuals following restrictive diets (e.g., vegan or vegetarian). Iron from plant sources (non-heme iron) has lower bioavailability and may not meet physiologic demands, especially in high-risk groups like pregnant women and children. - Poor Gastrointestinal Absorption
Conditions like celiac disease, inflammatory bowel disease (IBD), or post-bariatric surgery reduce the mucosal surface area or impair gastric acid production, which is essential for iron absorption. Even with adequate intake, malabsorption may lead to persistent anemia. - Chronic Blood Loss
A leading cause of IDA globally. In premenopausal women, heavy menstrual bleeding (menorrhagia) is a major contributor. In adults over 50, occult gastrointestinal bleeding due to peptic ulcers, malignancy, or NSAID use must be ruled out. Regular use of aspirin or anticoagulants can exacerbate the condition.
In IDA, both iron stores and serum iron levels are low, resulting in impaired hemoglobin synthesis and microcytic, hypochromic anemia. The body responds by increasing iron absorption and mobilization through suppression of hepcidin, but if the deficit persists, anemia worsens.
Diagnostic Challenges
AI and IDA often present with similar clinical symptoms (like fatigue, pallor, weakness) and comparable lab findings, including low hemoglobin and serum iron. However, traditional markers alone are not sufficient to reliably differentiate between them. Advanced diagnostic tools are now aiding in more accurate classification.
New Diagnostic Tools
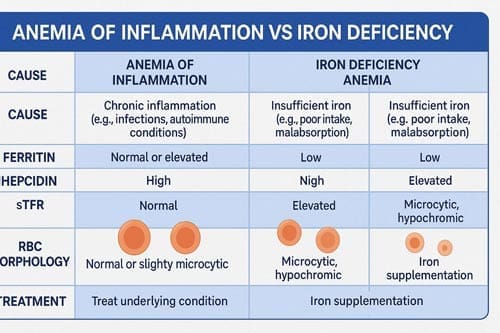
Ferritin reflects iron storage, but as an acute-phase reactant, it is elevated in inflammation.
- In IDA, ferritin is typically <30 ng/mL.
- In AI, ferritin may be normal or elevated despite insufficient iron availability.
To improve accuracy in inflammatory conditions, a cutoff of <100 ng/mL is often used. This adjustment accounts for elevated baseline ferritin due to inflammation and improves sensitivity in detecting true iron deficiency.
Hepcidin
Hepcidin is now considered a key biomarker in iron regulation:
- Low hepcidin is consistent with IDA, allowing for increased absorption and mobilization of iron.
- High hepcidin is a hallmark of AI and results in iron sequestration.
Measuring hepcidin can be particularly helpful when ferritin values are ambiguous. However, lack of standardized assays remains a challenge in clinical practice.
Other Complementary Markers
- Soluble Transferrin Receptor (sTfR):
Reflects the demand for iron in the bone marrow. Levels rise in IDA as erythropoietic activity increases to compensate for the deficiency. In contrast, sTfR remains normal or only mildly elevated in AI, making it a helpful tool in distinguishing between the two when ferritin values are inconclusive. - sTfR/log Ferritin Ratio (also known as the sTfR Index):
This combined marker offers a more nuanced interpretation of iron status. A high ratio (>2) suggests IDA, while a lower ratio is more consistent with AI or combined anemia. Its utility is especially important in mixed anemias, such as in elderly or chronically ill patients. - Reticulocyte Hemoglobin Content (CHr or Ret-He):
Measures the hemoglobin content of newly formed reticulocytes, providing a real-time snapshot of iron availability for erythropoiesis. A low CHr indicates recent or ongoing iron restriction, even before changes in traditional markers appear. It is especially useful in pediatric populations and in the early detection of treatment response.
Treatment Approaches
Managing Iron Deficiency Anemia (IDA)
Treatment aims to replenish iron stores and correct anemia:
- Oral iron therapy is first-line (e.g., ferrous sulfate, gluconate, or fumarate). Dosing typically ranges from 100–200 mg elemental iron daily. However, gastrointestinal side effects and poor absorption may limit effectiveness.
- Intravenous (IV) iron is indicated when oral iron is ineffective, poorly tolerated, or when rapid correction is needed (e.g., preoperative patients, late-stage pregnancy, or symptomatic anemia). Common IV formulations include ferric carboxymaltose, iron sucrose, and iron dextran.
Managing Anemia of Inflammation (AI)
Effective management requires addressing the underlying inflammatory condition. However, adjunct therapies are often necessary:
- Erythropoiesis-stimulating agents (ESAs): Used especially in CKD, cancer, or inflammatory diseases where endogenous erythropoietin is suppressed.
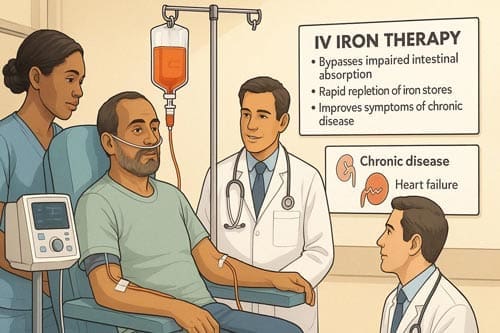
- IV iron therapy: Increasingly recognized for its ability to bypass hepcidin-mediated iron blockade and support red blood cell production, even in patients with normal ferritin levels.
- Anti-inflammatory treatment: Optimizing control of the primary disease can reduce hepcidin levels and partially reverse anemia.
The Role of IV Iron in Chronic Disease
Recent studies have shown that IV iron can be beneficial in treating AI associated with various chronic conditions:
- Chronic kidney disease: IV iron improves response to ESAs and may reduce ESA dosage requirements.
- Inflammatory bowel disease: IV iron can rapidly correct anemia and replenish iron stores.
- Heart failure: IV iron has been shown to improve symptoms and quality of life in patients with iron deficiency, with or without anemia.
- Cancer-related anemia: IV iron may enhance the efficacy of ESAs and reduce transfusion requirements.
Advantages of IV iron over oral iron in chronic disease include:
- Bypassing Impaired Intestinal Absorption:
In many chronic conditions, such as IBD or post-gastrointestinal surgery, inflammation or anatomical changes impair iron uptake. IV iron directly delivers iron to the bloodstream, overcoming absorption barriers and ensuring consistent bioavailability. - Rapid Repletion of Iron Stores:
IV iron allows for high-dose delivery in fewer infusions. Modern formulations such as ferric carboxymaltose or iron isomaltoside can deliver up to 1,000 mg in a single sitting, enabling full repletion in one or two visits. This is crucial in cases of severe anemia or when time-sensitive correction is needed (e.g., before major surgery). - Better Tolerability and Adherence:
Oral iron often causes gastrointestinal side effects such as constipation, nausea, and abdominal discomfort, leading to poor adherence. IV iron, particularly newer formulations, is better tolerated, making it a preferred option for patients with poor compliance or side effect sensitivity. - More Predictable Response:
Unlike oral iron, which may take weeks to show improvement, IV iron typically leads to measurable hemoglobin and ferritin improvements within 1–2 weeks. This makes it an efficient choice for healthcare systems needing rapid therapeutic outcomes.
However, potential risks such as allergic reactions and iron overload must be considered.
Challenges and Limitations
- Non-Standardized Hepcidin Assays:
Different laboratories use varied methods for measuring hepcidin, making comparisons difficult. Until standardized reference ranges are established, clinical interpretation must be cautious and often context-dependent. - Variable Ferritin Cutoffs:
Clinical guidelines differ on what constitutes “low,” “normal,” or “high” ferritin in inflammatory conditions. This lack of consistency can lead to misdiagnosis or under-treatment of IDA in patients with elevated inflammatory markers. - Lack of Long-Term Safety Data:
While short-term efficacy of IV iron is well-documented, there is limited data on long-term outcomes, especially with repeated courses in chronic disease management. More longitudinal studies are needed to understand cumulative risks. - Limited Awareness and Training:
Many clinicians are unfamiliar with newer iron markers or hesitate to use IV iron due to outdated perceptions of safety risks. Education and updated protocols are needed to facilitate evidence-based practice.
Future Directions
As our understanding of iron metabolism and the interplay between inflammation and anemia continues to evolve, several areas warrant further investigation:
- Personalized medicine approaches: Developing algorithms that incorporate multiple biomarkers, including genetic factors, to tailor diagnosis and treatment strategies for individual patients.
- Novel iron formulations: Research into new IV iron preparations with improved safety profiles and longer-lasting effects could enhance treatment options.
- Hepcidin antagonists: Drugs that block hepcidin’s action or reduce its production are being studied as potential treatments for anemia of inflammation.
- Combination therapies: Investigating the synergistic effects of combining IV iron with other treatments, such as ESAs or anti-inflammatory agents, may lead to more effective management strategies.
- Point-of-care testing: Developing rapid, accessible tests for hepcidin and other iron status markers could improve diagnosis and treatment monitoring in various clinical settings.
- Long-term outcomes: Conducting longitudinal studies to assess the impact of different treatment approaches on patient outcomes, quality of life, and overall health economics.
These areas of research hold promise for improving the diagnosis and management of both anemia of inflammation and iron deficiency anemia. As healthcare professionals, staying informed about these developments will be crucial for providing optimal care to patients with these complex conditions.

Conclusion
Distinguishing between anemia of inflammation and iron deficiency anemia is crucial for appropriate treatment. New diagnostic tools, particularly the use of hepcidin and refined interpretation of ferritin levels, offer improved accuracy in differentiating these conditions. IV iron therapy shows promise in managing anemia of inflammation across various chronic diseases. However, further research is needed to optimize diagnostic criteria and treatment protocols.
Frequently Asked Questions:
- Q: Can a patient have both iron deficiency anemia and anemia of inflammation?
A: Yes, these conditions can coexist, particularly in patients with chronic diseases. This scenario, often called “functional iron deficiency,” can be challenging to diagnose and manage.
- Q: Is IV iron safe for all patients with chronic diseases?
A: While IV iron is generally safe, it’s not suitable for everyone. Patients with active infections or a history of severe allergic reactions may not be candidates. Always assess individual risk-benefit profiles.
- Q: How quickly does IV iron work compared to oral iron?
A: IV iron typically leads to faster increases in hemoglobin levels and iron stores compared to oral iron, often showing improvements within 1-2 weeks.
- Q: Can hepcidin levels be used to guide iron therapy?
A: While promising, hepcidin-guided therapy is still largely experimental. More research is needed before it can be routinely used in clinical practice.
- Q: Are there any dietary changes that can help manage anemia of inflammation?
A: While diet alone cannot treat AI, a balanced diet rich in iron, vitamin C, and other nutrients supporting iron absorption and erythropoiesis can be beneficial as part of a comprehensive treatment plan.
References:
- Weiss, G., Ganz, T., & Goodnough, L. T. (2019). Anemia of inflammation. Blood, 133(1), 40-50.
- Girelli, D., Nemeth, E., & Swinkels, D. W. (2016). Hepcidin in the diagnosis of iron disorders. Blood, 127(23), 2809-2813.
- Auerbach, M., & Adamson, J. W. (2016). How we diagnose and treat iron deficiency anemia. American Journal of Hematology, 91(1), 31-38.
- Cappellini, M. D., Comin-Colet, J., de Francisco, A., Dignass, A., Doehner, W., Lam, C. S., … & Musallam, K. M. (2017). Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. American Journal of Hematology, 92(10), 1068-1078.
- Nemeth, E., & Ganz, T. (2014). Anemia of inflammation. Hematology/Oncology Clinics, 28(4), 671-681.
- Camaschella, C. (2019). Iron deficiency. Blood, 133(1), 30-39.
- Theurl, I., Aigner, E., Theurl, M., Nairz, M., Seifert, M., Schroll, A., … & Weiss, G. (2009). Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood, 113(21), 5277-5286.
- Anker, S. D., Comin Colet, J., Filippatos, G., Willenheimer, R., Dickstein, K., Drexler, H., … & Ponikowski, P. (2009). Ferric carboxymaltose in patients with heart failure and iron deficiency. New England Journal of Medicine, 361(25), 2436-2448.
- Wish, J. B. (2006). Assessing iron status: beyond serum ferritin and transferrin saturation. Clinical Journal of the American Society of Nephrology, 1(Supplement 1), S4-S8.
- Ganz, T. (2013). Systemic iron homeostasis. Physiological Reviews, 93(4), 1721-1741.
- Batchelor, E. K., Kapitsinou, P., Pergola, P. E., Kovesdy, C. P., & Jalal, D. I. (2020). Iron deficiency in chronic kidney disease: updates on pathophysiology, diagnosis, and treatment. Journal of the American Society of Nephrology, 31(3), 456-468.
- Gangat, N., & Wolanskyj, A. P. (2013). Anemia of chronic disease. Seminars in Hematology, 50(3), 232-238.
- Macdougall, I. C., Bircher, A. J., Eckardt, K. U., Obrador, G. T., Pollock, C. A., Stenvinkel, P., … & Fouque, D. (2016). Iron management in chronic kidney disease: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney International, 89(1), 28-39.
- Dignass, A. U., Gasche, C., Bettenworth, D., Birgegård, G., Danese, S., Gisbert, J. P., … & Vavricka, S. (2015). European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. Journal of Crohn’s and Colitis, 9(3), 211-222.
- Litton, E., Xiao, J., & Ho, K. M. (2013). Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta-analysis of randomised clinical trials. BMJ, 347, f4822