mTOR and Longevity: Rethinking the Role of Periodic Nutrient Stimulation Versus Chronic Suppression
Please like and subscribe if you enjoyed this video 🙂
Abstract
The mechanistic target of rapamycin (mTOR) pathway has emerged as a central regulator of aging and longevity, positioning itself at the intersection of nutrient sensing, cellular metabolism, and lifespan determination. Traditional paradigms have emphasized the benefits of chronic mTOR suppression through interventions such as caloric restriction and rapamycin administration. However, emerging evidence suggests a more nuanced relationship between mTOR signaling and longevity, challenging the conventional “less is more” approach. This analytical review examines the complex interplay between mTOR activation patterns and longevity outcomes, exploring the potential benefits of periodic nutrient stimulation versus chronic suppression. We synthesize current understanding of mTOR’s dual roles in cellular maintenance and aging, analyze the paradoxical effects of mTOR signaling in different contexts, and evaluate the implications of hormetic responses and autophagy regulation. The evidence suggests that optimal longevity may require a balanced approach incorporating both periods of mTOR activation and suppression, rather than chronic inhibition alone. This perspective has significant implications for developing age-modulating interventions and understanding the fundamental mechanisms underlying healthy aging.
Keywords: mTOR, longevity, aging, nutrient sensing, autophagy, hormesis, caloric restriction, rapamycin
Summary Table: Chronic Suppression vs. Periodic Activation of mTOR
| Feature | Chronic mTOR Suppression | Periodic mTOR Activation |
|---|---|---|
| Primary Strategy | Caloric restriction, low-protein diets, rapamycin | Protein cycling, refeeding after fasting, resistance exercise + amino acids |
| Goal | Promote longevity, reduce cellular growth | Enhance tissue repair, muscle growth, immune resilience |
| Typical Nutrients Involved | Low leucine, low methionine, plant-based proteins | Leucine-rich proteins (e.g., whey), BCAAs, high-protein meals |
| mTOR Pathway Activity | Suppressed long-term | Pulsed/spiked periodically |
| Longevity Impact (Animal Studies) | Extended lifespan in yeast, worms, flies, mice | Mixed results; unclear benefit for lifespan |
| Human Data (Longevity) | Lower IGF-1 and mTOR markers linked to reduced mortality; Blue Zones data support low protein | Lacks longitudinal studies; mostly performance-related research |
| Health Risks | Sarcopenia risk in elderly if suppression prolonged | Possible acceleration of aging or cancer risk if overused |
| Clinical Use Cases | Aging, cancer prevention, metabolic disease | Recovery, athletic performance, sarcopenia treatment |
| Controversies | May impair immune or regenerative responses if too suppressed | May negate anti-aging benefits if too frequent or high-intensity |
| Example Diets | Fasting, low-protein vegan, fasting-mimicking diet | Refeeding days, resistance training with protein intake |

1. Introduction
The quest to understand the fundamental mechanisms of aging has led researchers to identify several key pathways that regulate lifespan across species. Among these, the mechanistic target of rapamycin (mTOR) pathway has emerged as a central hub that integrates environmental signals with cellular responses to determine aging trajectories. The mechanistic target of rapamycin (mTOR) network is an evolutionary conserved signaling hub that senses and integrates environmental and intracellular nutrient and growth factor signals to coordinate basic cellular and organismal responses such as cell growth, proliferation, apoptosis, and inflammation depending on the individual cell and tissue. A growing list of evidence suggests that mTOR signaling influences longevity and aging. [1]
The traditional view of mTOR’s role in aging has been largely negative, with inhibition of the mTOR complex 1 (mTORC1) with rapamycin is currently the only known pharmacological treatment that increases lifespan in all model organisms studied. [2] This perspective has been reinforced by extensive research demonstrating that caloric restriction, which suppresses mTOR signaling, extends lifespan across multiple species. Furthermore, consistent with its role as a nutrient and growth factor sensor, decreased mTOR signaling reduces aging and thereby extends lifespan. [3] [4]
However, this paradigm faces significant challenges when considering the complex temporal dynamics of mTOR signaling and its multifaceted roles in cellular homeostasis. The mammalian/mechanistic target of rapamycin (mTOR) is a key component of cellular metabolism that integrates nutrient sensing with cellular processes that fuel cell growth and proliferation. Although the involvement of the mTOR pathway in regulating life span and aging has been studied extensively in the last decade, the underpinning mechanisms remain elusive. In this review, we highlight the emerging insights that link mTOR to various processes related to aging, such as nutrient sensing, maintenance of proteostasis, autophagy, mitochondrial dysfunction, cellular senescence, and decline in stem cell function. [5]
The central question addressed in this review is whether optimal longevity requires chronic mTOR suppression or whether periodic activation of mTOR, interspersed with periods of suppression, might provide superior outcomes. This analysis is particularly relevant given the growing recognition that mTOR signaling exhibits complex, context-dependent effects on cellular function and aging processes.
2. mTOR Signaling Architecture and Regulation
2.1 Structural Organization and Complexes
The mTOR kinase functions within two structurally and functionally distinct complexes: mTORC1 and mTORC2. mTOR operates within two functionally and structurally distinct complexes: mTORC1 and mTORC2. The two mTOR complexes share the components mLST8 and DEPTOR, while RAPTOR and PRAS40 are present exclusively in mTORC1. RICTOR, mSIN1, and Protor-1/2 are found exclusively within mTORC2. [6] This structural organization allows for differential regulation and distinct functional outputs that are crucial for understanding the pathway’s role in aging.
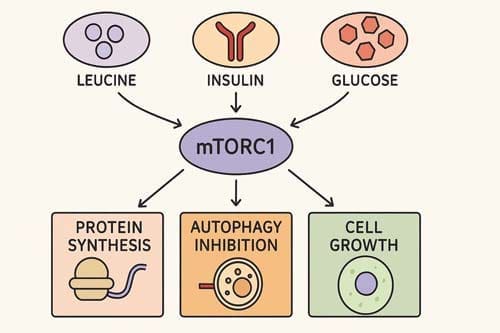
mTORC1 is primarily responsive to nutrient availability, particularly amino acids, and is directly inhibited by rapamycin. Growth factors or hormones (for example, insulin through IR) activate the PI3K/PDK1/AKT or ERK signaling pathways, which inactivate the TSC2 subunit of the TSC complex. Inactivation of the TSC complex upregulates the activity of RHEB, which in turn stimulates mTORC1. The activity of mTORC1 is also positively regulated by amino acid–mediated stimulation of the RAG complex of GTPases: Rag A/B and Rag C/D. [7]
2.2 Nutrient Sensing Mechanisms
The mTOR pathway’s function as a nutrient sensor is fundamental to its role in aging regulation. Presumably the most important stimulus that regulates mTORC1 activity is nutrient sufficiency, whereby amino acids play a predominant role. In fact, mTORC1 functions as a molecular sensor for amino acids, linking the cellular demand to the nutritional supply. [8] This sensing mechanism involves complex regulatory networks including the GATOR1/GATOR2 system and various amino acid sensors that detect specific amino acids such as leucine and arginine.
The temporal dynamics of nutrient sensing are particularly relevant for longevity outcomes. mTORC1 is suppressed under conditions where energy or glucose is limiting through AMPK signaling, which activates TSC2 and inhibits the mTORC1 subunit RAPTOR, and by hypoxia via the HIF-1α/REDD1 axis. mTORC1 orchestrates several anabolic processes via transcriptional or translational regulation or both. [9] This regulatory framework suggests that the pattern of nutrient availability, rather than simply the absolute level, may be critical for optimal aging outcomes.
2.3 Downstream Effector Pathways
mTORC1 regulates multiple downstream pathways that directly impact aging processes. mTORC1 controls protein synthesis in part through its two main effectors: S6K and 4E-BP1. mTORC1 also stimulates mitochondrial function, through 4E-BP1 and PGC-1α/YY1, and mitochondrial dynamics (via MTFP1). The control of mTORC1-mediated nucleotide synthesis is governed by S6K, ATF4, and PRPS2 (which is translationally regulated via 4E-BP/eIF4E), while lipid biosynthesis and adipogenesis are regulated by S6K and Lipin1. [10]
These diverse downstream effects highlight the complexity of mTOR’s role in aging, as they encompass processes that can be both beneficial and detrimental to longevity depending on the context and timing of activation.
3. The Paradox of mTOR in Cellular Senescence and Aging
3.1 mTOR and Cellular Senescence
The relationship between mTOR signaling and cellular senescence represents one of the most intriguing paradoxes in aging research. Deregulation of the nutrient sensitive mTOR signaling pathway has been recently involved in several age-related diseases, and pharmacological blockade of mTOR extends longevity in model organisms and in mice. Mechanistic studies in vitro have shed light on the role of mTOR-dependent growth signals in promoting senescence and exhaustion of quiescent stem cells, thus linking excess nutrients to tissue ageing. Novel findings add complexity to this theoretical framework, revealing that mTOR cooperates with autophagy to promote the “secretory phenotype” of senescent cells and the release of factors known to contribute to defective renewal and dysfunction of aging tissues. [11]
This dual role of mTOR in senescence presents a complex picture. The choice between p53-induced senescence and quiescence is determined in part by the mTOR pathway. [12] While chronic mTOR activation can promote senescence, complete mTOR inhibition may not be beneficial either, as it can impair cellular maintenance and repair mechanisms.
3.2 The Senescence-Associated Secretory Phenotype (SASP)
The role of mTOR in regulating the SASP adds another layer of complexity to its effects on aging. Novel findings add complexity to this theoretical framework, revealing that mTOR cooperates with autophagy to promote the “secretory phenotype” of senescent cells and the release of factors known to contribute to defective renewal and dysfunction of aging tissues. Thus, both cell autonomous and cell non-autonomous mechanisms link unchecked mTOR activity to cell senescence and by extension to the aging process. [13]
This suggests that the effects of mTOR on aging extend beyond individual cells to influence tissue-level aging through paracrine signaling mechanisms. The implications for therapeutic interventions are significant, as simple mTOR inhibition may not address all aspects of age-related dysfunction.
3.3 Aberrant mTOR Activation in Aging
Paradoxically, several studies have reported increased mTOR signaling in aged tissues, challenging the simple model of chronic mTOR suppression for longevity. Unexpected activation of mTOR signaling, measured by ribosomal S6 phosphorylation or ribosomal S6 kinase (p70S6K) activity, has been reported in aging-related settings. Evidence of elevated mTOR activity has been reported in the heart and muscle tissue in aged mice and humans, mouse models of progeria, and senescent human fibroblasts. We explore these reports and the possibility that activation of the mTOR/p70S6K kinase pathway may represent a ROS-mediated response to mitochondrial stress leading to the activation of senescence. [14]
This aberrant activation suggests that the relationship between mTOR and aging is more complex than previously thought, with both excessive activation and complete suppression potentially being detrimental.
4. Caloric Restriction and mTOR: Temporal Dynamics
4.1 Caloric Restriction Mechanisms
Caloric restriction (CR) has been extensively studied as a longevity intervention, with mTOR suppression considered a key mechanism. Caloric restriction (CR), reduction of calorie intake to a level that does not compromise overall health, has been considered as being one of the most promising dietary interventions to extend lifespan in humans. Although it is straightforward, continuous reduction of calorie or food intake is not easy to practice in real lives of humans. Recently, fasting-related interventions such as intermittent fasting (IF) and time-restricted feeding (TRF) have emerged as alternatives of CR. [15]
The mechanisms underlying CR’s effects on longevity involve multiple pathways, with mTOR being a central hub. The different pathways regulated by CR include:(1) AMP-activated protein kinase (AMPK), which involves PGC-1α, SIRT1, and SIRT3. AMPK also effects myocyte enhancer factor 2 (MEF2), peroxisome proliferator-activated receptor delta, and peroxisome proliferator-activated receptor alpha, which are involved in mitochondrial biogenesis and lipid oxidation; (2) Forkhead box transcription factor’s signaling is related to the DNA repair, lipid metabolism, protection of protein structure, autophagy, and resistance to oxidative stress; (3) Mammalian target of rapamycin (mTOR) signaling, which involves key factors, such as S6 protein kinase-1 (S6K1), mTOR complex-1 (mTORC1), and 4E-binding protein (4E-BP). [16]
4.2 Intermittent Fasting and Periodic Nutrient Stimulation
The emergence of intermittent fasting (IF) as an alternative to continuous caloric restriction provides insights into the potential benefits of periodic nutrient stimulation. Different dietary interventions by CR and IF result in similar molecular and physiological changes that promote longevity in model organisms. Patterns of individual dietary, metabolic, molecular, and physiological parameters can be different depending on the types of CR and IF as well as the animal models. [17]
Research comparing continuous CR with intermittent approaches has revealed important differences. Chronic calorie restriction (CCR) prevents mammary tumor (MT) development in rodents. We reported that intermittent calorie restriction (ICR) provides greater protection than CCR in MMTV-TGF-α mice. The mammalian target of rapamycin (mTOR) pathway is involved in MT development. [18] This suggests that the temporal pattern of nutrient availability may be as important as the absolute level of restriction.
4.3 Time-Dependent Effects of Caloric Restriction
The temporal dynamics of mTOR regulation under caloric restriction are complex and tissue-specific. CR effects on mTORC1 and mTORC2 activities were time-of-day dependent. CR induced mTORC1 activity at one time, reduced at two times and has no effect during other times. CR induced mTORC2 activity at one time of the day and has no effects at other times. [19]
This temporal variability suggests that the effects of CR on mTOR signaling are not uniform but depend on circadian rhythms and other temporal factors. Circadian clocks are implemented in the regulation of mTOR signaling in mammals and mechanisms of CR. [20] These findings have important implications for understanding how periodic nutrient stimulation might be optimized for longevity benefits.

5. Autophagy and the mTOR-Longevity Connection
5.1 Autophagy as a Longevity Mechanism
Autophagy, the cellular process of self-digestion and recycling, is intricately linked to both mTOR signaling and longevity. During aging, the efficiency of autophagic degradation declines and intracellular waste products accumulate. In Caenorhabditis elegans, there is clear evidence that lifespan is linked to the capacity to regulate autophagy. Recent studies have revealed that the same signaling factors regulate both aging and autophagocytosis, thus highlighting the role of autophagy in the regulation of aging and age-related degenerative diseases. [21]
The relationship between autophagy and mTOR is generally described as inhibitory, with mTOR suppression promoting autophagy. In particular, the repressive effects of mammalian target of rapamycin (mTOR) and the role of Beclin 1, a repressor or activator of caloric restriction (CR), which is also called dietary restriction, is the only strategy known to extend lifespan universally in a wide range of organisms from yeast to non-human primates. However, several studies have demonstrated that autophagic degradation increases during caloric restriction. Commonly, this is linked to the downregulation of the mTOR pathway, both in CR and during times of limited energy availability. [22] [23]
5.2 The Complexity of mTOR-Autophagy Interactions
However, the relationship between mTOR and autophagy is more nuanced than simple inhibition. Although previous studies provided evidence that mTOR signaling was the most important inhibitor of autophagy, our results here suggest that the inhibitory role of mTOR signaling is not critical for the age-dependent deficit of autophagy during normal brain aging. [24] This suggests that other factors may be more important for autophagy regulation during aging.
Furthermore, the timing and context of mTOR-autophagy interactions appear critical. Increasing lines of evidence suggest that autophagy is required for many mechanisms that mediate lifespan extension, such as caloric restriction, in various organisms. These results raise the exciting possibility that autophagy may play an important role in combating the adverse effects of aging in the heart. [25]
5.3 Stem Cell Function and Autophagy
The role of autophagy in stem cell function provides another perspective on the mTOR-longevity relationship. Studies suggest that autophagy modulates various cellular processes and states of adult SCs, including quiescence, proliferation, self-renewal, and differentiation. The primary role of autophagy in these contexts is to sustain homeostasis, withstand stressors, and supply energy. Notably, the dysfunction of adult SCs during aging is correlated with a decline in autophagic activity, suggesting that autophagy is also involved in SC- and aging-associated disorders. [26]
This connection between autophagy, stem cell function, and aging suggests that optimal longevity strategies must consider the complex interplay between these processes rather than focusing solely on mTOR suppression.
6. Hormesis and Periodic Stress Response
6.1 The Hormesis Paradigm
Hormesis, the beneficial effects of mild stress, provides a framework for understanding how periodic activation of stress response pathways, including mTOR, might contribute to longevity. Hormesis in aging is represented by mild stress-induced stimulation of protective mechanisms in cells and organisms resulting in biologically beneficial effects. Although the extent of immediate hormetic effects after exposure to a particular stress may only be moderate, the chain of events following initial hormesis leads to biologically amplified effects that are much larger, synergistic and pleiotropic. A consequence of hormetic amplification is an increase in the homeodynamic space of a living system in terms of increased defence capacity and reduced load of damaged macromolecules. [27] [28]
The hormesis concept challenges the simple “less is more” approach to mTOR and aging. Hormetic strengthening of the homeodynamic space provides wider margins for metabolic fluctuation, stress tolerance, adaptation and survival. Hormesis thus counter-balances the progressive shrinkage of the homeodynamic space, which is the ultimate cause of aging, diseases and death. Healthy aging may be achieved by hormesis through mild and periodic, but not severe or chronic, physical and mental challenges, and by the use of nutritional hormesis incorporating mild stress-inducing molecules called hormetins. [29]
6.2 Hormesis and mTOR Signaling
The relationship between hormesis and mTOR signaling is complex and depends on the type and intensity of stress. Instead, aging is driven by overactivated signal-transduction pathways including the TOR (Target of Rapamycin) pathway. A diverse group of hormetic conditions can be divided into two groups. Nutrients (food), growth factors, cytokines, insulin and hormones activate the nutrient-sensing TOR (Target of Rapamycin) pathway, which promotes growth and then aging, causing age-related diseases. [30]
However, the hormetic effects of mTOR signaling can be categorized into different types. Hormesis type A inhibits TOR thus slowing down aging. Hormesis type B increases aging-tolerance and tolerance to complications of age-related diseases. [31] This suggests that both mTOR inhibition and activation can have hormetic effects depending on the context and timing.
6.3 Rapamycin and Hormetic Responses
Rapamycin itself exhibits hormetic properties, with different effects at different concentrations. Rapamycin, at high and low doses, has biphasic effects in cells—a phenomenon called hormesis. While the drug is toxic to cells at elevated doses, it can reportedly augment longevity at lower doses. Low concentration of rapamycin leads to incomplete inhibition in the function of mTOR, in contrast to the putative complete mTOR inhibition at higher doses.
This hormetic response to rapamycin provides insights into how periodic, rather than chronic, mTOR modulation might be optimal for longevity. It is evident that rapamycin demonstrates its hormetic nature by eliciting significant anti-aging effects in cells at low doses, by partial inhibition of mTOR function and associated modulation of the mTOR–mitochondria cross-talk.

7. Metabolic Flexibility and Aging
7.1 The Importance of Metabolic Flexibility
Metabolic flexibility, the ability to switch between different metabolic states, is increasingly recognized as important for healthy aging. “Metabolic aging” refers to the gradual decline in cellular metabolic function across various tissues due to defective hormonal signaling, impaired nutrient sensing, mitochondrial dysfunction, replicative stress, and cellular senescence. While this process usually corresponds with chronological aging, the recent increase in metabolic diseases and cancers occurring at younger ages in humans suggests the premature onset of cellular fatigue and metabolic aging. Autophagy, a cellular housekeeping process facilitated by lysosomes, plays a crucial role in maintaining tissue rejuvenation and health. [32]
The concept of metabolic flexibility suggests that alternating between periods of mTOR activation and suppression might be more beneficial than chronic suppression. Autophagy regulates nutrient sensing, mitochondrial function, cellular senescence, and protein homeostasis. Autophagy modulation may help to promote health aging. [33]
7.2 Age-Related Changes in mTOR Signaling
The effects of aging on mTOR signaling are tissue-specific and context-dependent. Calorie restriction (CR) reduced phosphorylation of mTOR and S6K and decreased the content of FOXO3a and ubiquitinated proteins in skeletal muscles in middle-aged rats. CR did not change these pathways in skeletal muscles of young and adult rats. Unlike younger rats, CR decreased the content of phosphorylated mTOR, S6K, phosphorylated S6K, FOXO3a, and ubiquitinated proteins in middle-aged rats. [34] [35]
This age-dependent response suggests that the optimal mTOR signaling pattern may change with age, with different strategies being appropriate for different life stages.
7.3 Nutrient Sensing and Metabolic Health
The role of mTOR in nutrient sensing has implications for metabolic health and longevity. Conversely, nutritional amino acid overload has been tightly linked to aging and diseases, such as cancer, type 2 diabetes and obesity. Similar effects can also be recapitulated by mutations in upstream mTORC1 regulators, thus establishing a tight connection between mTORC1 signaling and aging. Although the role of growth factor signaling upstream of mTORC1 in aging has been investigated extensively, the involvement of signaling components participating in the nutrient sensing branch is less well understood. [36]
This suggests that the pattern of nutrient availability, rather than simply the quantity, may be crucial for optimal aging outcomes.
8. Tissue-Specific Considerations
8.1 Brain and Neurological Function
The brain presents unique challenges for understanding mTOR’s role in aging. In multicellular organisms, mTOR regulates cell growth and metabolism in response to nutrients, growth factors and cellular energy conditions. Growing studies highlight that disturbance in mTOR signalling in the brain affects multiple pathways including glucose metabolism, energy production, mitochondrial function, cell growth and autophagy. All these events are key players in age-related cognitive decline such as development of Alzheimer disease (AD). [37]
The complexity of mTOR signaling in the brain suggests that simple inhibition may not be optimal. However, other data show that mTOR activation is indispensable for dendritic morphogenesis, synaptic plasticity, and the consolidation of long-term memories. Outwardly, these are contradictory results. Therefore, the role of mTOR and its dependent autophagy in the healthy brain during aging do need to be researched. [38]
8.2 Cardiovascular System
The cardiovascular system also demonstrates complex relationships between mTOR signaling and aging. Autophagy and autophagic flux are generally decreased in aging hearts, and murine autophagy loss-of-function models develop exacerbated cardiac dysfunction that is accompanied by the accumulation of misfolded proteins and dysfunctional organelles. On the contrary, stimulation of autophagy generally improves cardiac function in mouse models of protein aggregation by removing accumulated misfolded proteins, dysfunctional mitochondria, and damaged DNA, thereby improving the overall cellular environment and alleviating aging-associated pathology in the heart. [39]
8.3 Muscle and Metabolic Tissues
Skeletal muscle provides another example of tissue-specific mTOR effects. One level on which a CR diet promotes longevity is at the level of stem cells. Research from the Sabatini and Wagers laboratories has demonstrated that CR works at the cellular level to regulate stem cell proliferation. In muscle, the Wagers laboratory found that CR enhances muscle stem cell availability and activity, increasing mitochondrial abundance. [40]
However, the effects of CR on muscle may be age-dependent, with different responses in young versus aged animals. However, both the Wagers and Sabatini laboratory studies were conducted in young mice on a CR diet for a relatively short period of time, and it has yet to be shown if these effects continue to occur in aged animals, or if they contribute to organismal longevity. [41]
9. Therapeutic Implications and Future Directions
9.1 Rethinking mTOR-Targeted Therapies
The evidence presented suggests that therapeutic approaches to aging should move beyond simple mTOR inhibition toward more nuanced strategies that consider temporal patterns and tissue-specific requirements. Inhibition of this pathway extends lifespan in model organisms and confers protection against a growing list of age-related pathologies. Characterized inhibitors of this pathway are already clinically approved, and others are under development. Although adverse side effects currently preclude use in otherwise healthy individuals, drugs that target the mTOR pathway could one day become widely used to slow ageing and reduce age-related pathologies in humans. [42]
9.2 Personalized Approaches to Longevity
The complexity of mTOR signaling suggests that personalized approaches to longevity interventions may be necessary. By understanding the intricate interplay between diet and molecular pathways, we can develop personalized dietary strategies that not only prevent age-related diseases, but also promote overall health and well-being throughout the aging process. [43]
9.3 Integration with Other Longevity Pathways
Future research should consider how mTOR signaling integrates with other longevity pathways. AMPK signaling is involved in the regulation of all these characteristics via an integrated signaling network. Many studies with lower organisms have revealed that increased AMPK activity can extend the lifespan. Experiments in mammals have demonstrated that AMPK controls autophagy through mTOR and ULK1 signaling which augment the quality of cellular housekeeping. [44]
10. Synthesis and Critical Analysis
10.1 Reconciling Contradictory Evidence
The evidence presented reveals a complex picture of mTOR’s role in aging that cannot be reduced to simple inhibition or activation. The apparent contradictions in the literature may be resolved by considering several key factors:
-
Temporal dynamics: The timing and duration of mTOR activation or suppression appear to be crucial determinants of outcome.
-
Tissue specificity: Different tissues have varying requirements for mTOR signaling, and these requirements may change with age.
-
Dose-response relationships: The effects of mTOR modulation appear to follow hormetic dose-response curves, with optimal effects at intermediate levels of activity.
-
Integration with other pathways: mTOR functions as part of a complex network of signaling pathways, and its effects cannot be understood in isolation.
10.2 The Case for Periodic Nutrient Stimulation
The evidence suggests several potential advantages of periodic nutrient stimulation over chronic suppression:
-
Maintenance of cellular function: Periodic mTOR activation may be necessary for maintaining essential cellular processes such as protein synthesis and mitochondrial function.
-
Stem cell maintenance: Some level of mTOR activity appears necessary for stem cell function and tissue regeneration.
-
Metabolic flexibility: Alternating between fed and fasted states may promote metabolic flexibility and resilience.
-
Hormetic responses: Periodic activation may trigger beneficial stress responses that enhance cellular resilience.
10.3 Limitations and Future Research Needs
Several limitations in current research need to be addressed:
-
Temporal resolution: Most studies examine chronic interventions and may miss important temporal dynamics.
-
Tissue-specific effects: More research is needed on how mTOR signaling differs across tissues and how these differences change with age.
-
Translation to humans: Most research has been conducted in model organisms, and translation to humans remains challenging.
-
Mechanistic understanding: The precise mechanisms underlying the beneficial effects of periodic nutrient stimulation need further elucidation.

11. Conclusion
The relationship between mTOR signaling and longevity is far more complex than initially appreciated. While chronic mTOR suppression through caloric restriction or rapamycin treatment can extend lifespan in model organisms, emerging evidence suggests that optimal longevity may require a more nuanced approach that incorporates both periods of mTOR activation and suppression.
The concept of periodic nutrient stimulation, exemplified by intermittent fasting and other temporal dietary interventions, offers a promising alternative to chronic suppression. This approach may better preserve essential cellular functions while still providing the longevity benefits associated with mTOR pathway modulation.
Key insights from this analysis include:
-
Temporal patterns matter: The timing and duration of mTOR signaling appear to be as important as the absolute level of activity.
-
Context dependency: The effects of mTOR modulation are highly dependent on tissue type, age, and other contextual factors.
-
Hormetic responses: Mild, periodic activation of mTOR may trigger beneficial hormetic responses that enhance cellular resilience.
-
Integration with other pathways: mTOR functions as part of a complex network, and interventions must consider these interactions.
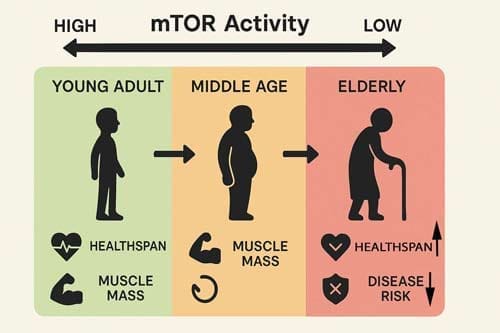
The implications for aging research and therapeutic development are significant. Rather than seeking to maximally suppress mTOR signaling, future interventions should focus on optimizing the temporal patterns of mTOR activity to maximize longevity benefits while minimizing detrimental effects.
This paradigm shift has important implications for understanding the fundamental mechanisms of aging and developing more effective interventions for healthy aging. As our understanding of mTOR signaling complexity continues to evolve, it becomes increasingly clear that the path to optimal longevity lies not in simple suppression, but in the careful orchestration of activation and suppression patterns that maintain cellular homeostasis while promoting healthy aging.
Future research should focus on defining optimal temporal patterns of mTOR signaling for different tissues and life stages, developing interventions that can precisely control these patterns, and understanding how these approaches can be integrated with other longevity strategies to maximize healthspan and lifespan.

Key References
Anisimov, V. N., et al. (2010). Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle, 9(21), 4050-4057.
Calabrese, E. J., & Baldwin, L. A. (2002). Defining hormesis. Human & Experimental Toxicology, 21(2), 91-97.
Cornu, M., Albert, V., & Hall, M. N. (2013). mTOR in aging, metabolism, and cancer. Current Opinion in Genetics & Development, 23(1), 53-62.
Harrison, D. E., et al. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature, 460(7253), 392-395.
Johnson, S. C., Rabinovitch, P. S., & Kaeberlein, M. (2013). mTOR is a key modulator of ageing and age-related disease. Nature, 493(7432), 338-345.
Lamming, D. W., et al. (2012). Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science, 335(6076), 1638-1643.
Mattson, M. P., et al. (2017). Intermittent metabolic switching, neuroplasticity and brain health. Nature Reviews Neuroscience, 18(2), 63-75.
Papadopoli, D., et al. (2019). mTOR as a central regulator of lifespan and aging. F1000Research, 8, F1000 Faculty Rev-998.
Rattan, S. I. (2008). Hormesis in aging. Ageing Research Reviews, 7(1), 63-78.
Saxton, R. A., & Sabatini, D. M. (2017). mTOR signaling in growth, metabolism, and disease. Cell, 168(6), 960-976.
Weichhart, T. (2018). mTOR as regulator of lifespan, aging, and cellular senescence: A mini-review. Gerontology, 64(2), 127-134.
References
[1] mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/29190625/
[2] mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/29190625/
[3] mTOR in aging, metabolism, and cancer – ScienceDirect – www.sciencedirect.comhttps://www.sciencedirect.com/s
cience/article/abs/pii/S0959437X12001499
[4] mTOR in aging, metabolism, and cancer – ScienceDirect – www.sciencedirect.comhttps://www.sciencedirect.com/
science/article/abs/pii/S0959437X12001499
[5] From growing to secreting: new roles for mTOR in aging cells – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/21720215/
[6] From growing to secreting: new roles for mTOR in aging cells – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/21720215/
[7] From growing to secreting: new roles for mTOR in aging cells – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/21720215/
[8] mTOR and autophagy in normal brain aging and caloric restriction ameliorating age-related cognition deficits – ScienceDirect – www.sciencedirect.comhttps://www.sciencedirect.com/
science/article/abs/pii/S0166432814000722
[9] Lipid-induced mTOR activation in rat skeletal muscle reversed by exercise and 5′-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/19574345/
[10] Lipid-induced mTOR activation in rat skeletal muscle reversed by exercise and 5′-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/19574345/
[11] mTOR favors senescence over quiescence in p53-arrested cells – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/20603524/
[12] Targeting metabolism in cellular senescence, a role for intervention – ScienceDirect – www.sciencedirect.comhttps://www.sciencedirect.com/
science/article/abs/pii/S0303720716303550
[13] mTOR favors senescence over quiescence in p53-arrested cells – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/20603524/
[14] mTOR signaling in aging and neurodegeneration: At the crossroad between metabolism dysfunction and impairment of autophagy – ScienceDirect – www.sciencedirect.comhttps://www.sciencedirect.com/
science/article/abs/pii/S0969996115000868
[15] Lifespan-extending caloric restriction or mTOR inhibition impair adaptive immunity of old mice by distinct mechanisms – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/25424641/
[16] mTOR as a central regulator of lifespan and aging – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/31316753/
[17] Lifespan-extending caloric restriction or mTOR inhibition impair adaptive immunity of old mice by distinct mechanisms – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/25424641/
[18] Molecular Mechanisms of Healthy Aging: The Role of Caloric Restriction, Intermittent Fasting, Mediterranean Diet, and Ketogenic Diet-A Scoping Review – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/39275194/
[19] Downregulation of mTOR Signaling Increases Stem Cell Population Telomere Length during Starvation of Immortal Planarians – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/31353226/
[20] Downregulation of mTOR Signaling Increases Stem Cell Population Telomere Length during Starvation of Immortal Planarians – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/31353226/
[21] Growth or death? Control of cell destiny by mTOR and autophagy pathways – ScienceDirect – www.sciencedirect.comhttps://www.sciencedirect.com/
science/article/abs/pii/S0079610723000925
[22] Growth or death? Control of cell destiny by mTOR and autophagy pathways – ScienceDirect – www.sciencedirect.comhttps://www.sciencedirect.com/
science/article/abs/pii/S0079610723000925
[23] Growth or death? Control of cell destiny by mTOR and autophagy pathways – ScienceDirect – www.sciencedirect.comhttps://www.sciencedirect.com/
science/article/abs/pii/S0079610723000925
[24] Autophagy – An Emerging Anti-Aging Mechanism – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/23750326/
[25] Autophagy in adult stem cell homeostasis, aging, and disease therapy – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/40208372/
[26] mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/29190625/
[27] Hormesis and aging in Caenorhabditis elegans – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/17067771/
[28] Hormesis and aging in Caenorhabditis elegans – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/17067771/
[29] Hormesis and aging in Caenorhabditis elegans – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/17067771/
[30] Hormesis in aging – ScienceDirect – www.sciencedirect.comhttps://www.sciencedirect.com/
science/article/abs/pii/S1568163707000360
[31] Hormesis in aging – ScienceDirect – www.sciencedirect.comhttps://www.sciencedirect.com/
science/article/abs/pii/S1568163707000360
[32] mTOR Signaling in Growth Control and Disease – ScienceDirect – www.sciencedirect.comhttps://www.sciencedirect.com/
science/article/pii/S0092867412003510
[33] mTOR Signaling in Growth Control and Disease – ScienceDirect – www.sciencedirect.comhttps://www.sciencedirect.com/
science/article/pii/S0092867412003510
[34] mTOR as a senescence manipulation target: A forked road – ScienceDirect – www.sciencedirect.comhttps://www.sciencedirect.com/
science/article/abs/pii/S0065230X2100018X
[35] mTOR as a senescence manipulation target: A forked road – ScienceDirect – www.sciencedirect.comhttps://www.sciencedirect.com/
science/article/abs/pii/S0065230X2100018X
[36] mTOR and autophagy in normal brain aging and caloric restriction ameliorating age-related cognition deficits – ScienceDirect – www.sciencedirect.comhttps://www.sciencedirect.com/
science/article/abs/pii/S0166432814000722
[37] Aging and Autophagy in the Heart – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/27174950/
[38] Autophagy – An Emerging Anti-Aging Mechanism – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/23750326/
[39] Autophagy in adult stem cell homeostasis, aging, and disease therapy – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/40208372/
[40] Calorie Restriction-Regulated Molecular Pathways and Its Impact on Various Age Groups: An Overview – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/35451872/
[41] Calorie Restriction-Regulated Molecular Pathways and Its Impact on Various Age Groups: An Overview – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/35451872/
[42] mTOR is a key modulator of ageing and age-related disease – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/23325216/
[43] Mechanisms of Lifespan Regulation by Calorie Restriction and Intermittent Fasting in Model Organisms – PubMed – pubmed.ncbi.nlm.nih.govhttps://pubmed.ncbi.nlm.nih.gov/32344591/
[44] Growth or death? Control of cell destiny by mTOR and autophagy pathways – ScienceDirect – www.sciencedirect.comhttps://www.sciencedirect.com/science/
article/abs/pii/S0079610723000925
