Remyelination in MS: Scientists Uncover Promising Treatment Pathway
Introduction
Multiple sclerosis (MS) research has entered a transformative phase with the emergence of two promising therapies aimed at restoring myelin, the protective sheath around nerve fibers that is progressively damaged in MS. Affecting an estimated 2.5 million people worldwide, MS leads to neurological impairments including motor dysfunction, vision loss, and, in severe cases, paralysis and reduced life expectancy.
While current treatments focus on modulating immune activity to reduce relapses, they do not directly address the repair of myelin. However, recent advances offer new hope by targeting the underlying mechanisms of remyelination.
One of the most notable developments is PIPE-307, a small molecule developed by UC San Francisco and Contineum Therapeutics. PIPE-307 targets the M1R receptor to stimulate oligodendrocyte precursor cells (OPCs) involved in myelin repair. In preclinical studies, the compound reversed myelin loss in mouse models of MS. Following two Phase I trials that confirmed safety, PIPE-307 is now undergoing Phase II clinical evaluation.
Another breakthrough is ESI1, a novel protein function inhibitor that promotes remyelination by enhancing myelin production in both MS mouse models and cultured human brain cells. ESI1 has demonstrated nearly five times greater efficacy than comparable compounds in promoting oligodendrocyte-driven myelination.
These therapies are grounded in growing insights into the biology of oligodendrocytes, the cells responsible for myelin production and the discovery that inhibitory signals suppressing myelin repair can be modulated or reversed. This represents a significant step forward in the field.
Importantly, these remyelination agents may complement existing immunomodulatory therapies. Used in combination, they have the potential not only to prevent new damage but also to repair existing lesions addressing the critical unmet need of functional recovery in MS.
Understanding Myelin Loss in MS and Its Consequences
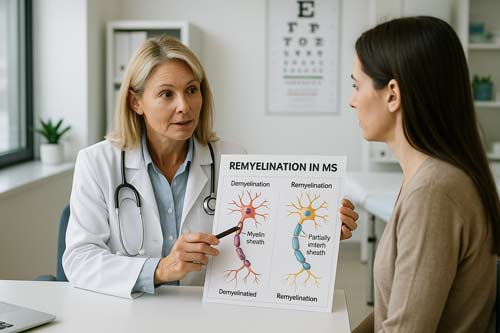
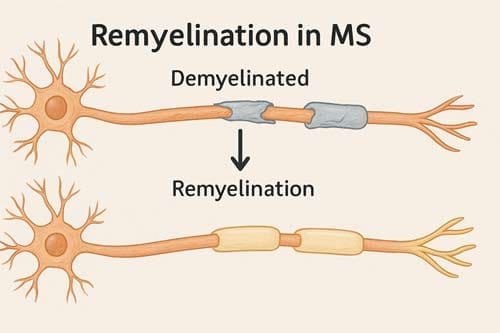
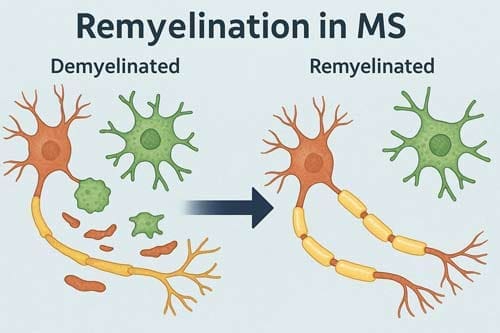
At the core of multiple sclerosis pathology lies the autoimmune damage to oligodendrocytes and the resulting demyelination. Myelin sheaths serve as critical insulators for axons, ensuring safe and reliable impulse propagation essential for normal neural function. Their loss leads directly to reduced axonal integrity which, over time, results in neuronal fallout and fixed functional deficits [1].
Oligodendrocyte Dysfunction and Axonal Exposure
Demyelinated axons face immediate consequences beyond just losing their insulating properties. Contrary to earlier assumptions that axons were largely spared in MS, recent evidence shows that axonal injury occurs early in the disease process, even before overt demyelination [2]. When myelin is damaged, axons lose both physical protection and vital metabolic support. Myelinating oligodendrocytes provide more than mere electrical insulation they deliver essential energy substrates to axons through the monocarboxylate transporter 1 (MCT1) [3].
Paradoxically, the normally symbiotic relationship between axons and oligodendrocytes can become fatal during inflammatory attacks. In active MS lesions, two primary types of axonal pathology emerge:
- Axons with intra-axonal organelle accumulations (potentially reversible)
- Axons with highly condensed axoplasm (irreversible damage) [2]
Moreover, axonal damage directly correlates with lesion activity, supporting the hypothesis that inflammatory mediators produced by immune or glial cells play a significant role in early axonal injury [4].
Why Natural Remyelination Fails in MS Patients
Despite the presence of oligodendroglial precursor cells (OPCs) and neural stem cells in the adult brain, remyelination remains inefficient in MS patients. These cells can theoretically differentiate into mature oligodendrocytes capable of remyelinating demyelinated axons. Nevertheless, they are prevented from effectively differentiating by various molecular mechanisms [1].
Remyelination failure increases with age and disease duration due to:
- Reduced myelin debris clearance by microglia
- Diminished secretion of factors promoting OPC differentiation [1]
- Overexpression of inhibitors including PSA-NCAM, LINGO-1, and Jagged [1]
Furthermore, in chronic lesions, OPCs are present albeit in reduced numbers and unevenly distributed, whereas mature oligodendrocytes are almost completely absent [5]. This observation supports the concept of impaired oligodendroglial differentiation as a key factor contributing to limited remyelination in chronic MS [5].
Understanding these mechanisms provides deep insights for developing remyelination therapies that could potentially address the fundamental challenges in MS treatment.
Targeting M1R: The Science Behind PIPE-307
The discovery that muscarinic receptor signaling inhibits oligodendrocyte precursor cell (OPC) differentiation has opened promising avenues for remyelination treatment strategies. Recent advances have specifically identified the M1 muscarinic acetylcholine receptor (M1R) as a crucial negative regulator of oligodendrocyte maturation and myelination [6].
Muscarinic Receptor Blockade and OPC Activation
Researchers initially identified M1R as a therapeutic target through unbiased screening of FDA-approved compounds, which revealed that antimuscarinic drugs promoted oligodendrocyte differentiation [6]. Throughout the central nervous system, OPCs remain abundant even in MS patients, yet they fail to mature into myelin-producing oligodendrocytes. Blockade of M1R appears to release these cells from their stalled state, enabling myelin production.
In laboratory experiments, acetylcholine inhibited OPC differentiation with an IC50 of 57.7 nM [7]. Consequently, researchers hypothesized that antagonizing M1R could reverse this inhibition and promote remyelination. This insight led to the development of PIPE-307, an orally bioavailable, brain-penetrant small molecule specifically targeting M1R.
MT7 Toxin for M1R Localization in Human and Mouse Models
To precisely identify M1R’s location in the brain, scientists utilized MT7, a peptide from green mamba snake venom with exquisite selectivity for M1R in the subnanomolar range [8][9]. This breakthrough approach allowed researchers to:
- Confirm M1R protein presence on OPCs in both mouse and human brain tissue
- Specifically localize M1R on OPCs at borders between MS lesions and normal-appearing white matter [10]
- Validate M1R as a remyelination target in human MS tissue
By conjugating a fluorophore to MT7, researchers created a highly selective probe that revealed rings of OPCs gathering around damage in MS lesions [11], providing compelling evidence for M1R as a therapeutic target.
PIPE-307 vs Clemastine: Selectivity and Efficacy Comparison
While clemastine (a first-generation antihistamine) demonstrated antimuscarinic properties, it lacked selectivity for M1R. In contrast, PIPE-307 shows remarkable specificity:
- PIPE-307 binds to human M1R with a Ki of 4.6 nM [6][7]
- It maintains >10-fold selectivity against other muscarinic receptor subtypes (M2-M5) [6]
- In functional assays, it inhibits acetylcholine-induced calcium mobilization with an IC50 of 3.8 nM [6]
PIPE-307 induces OPC differentiation with an EC50 of 38.6 nM [12], promoting myelin basic protein expression comparable to established positive controls [6]. After clearing Phase I trials demonstrating safety, PIPE-307 has advanced to Phase II clinical trials in relapsing-remitting MS patients [13].
ESI1 and Epigenetic Reprogramming of Oligodendrocytes
Recent studies examining the role of epigenetics in multiple sclerosis have revealed intriguing mechanisms underlying remyelination failure that offer novel therapeutic approaches. The compound ESI1 (epigenetic-silencing-inhibitor-1) represents a promising advance in this field, demonstrating remarkable efficacy in activating dormant oligodendrocytes through targeted epigenetic modifications.
Histone Modifications: H3K27ac vs H3K27me3 in MS Lesions
Analysis of autopsy tissues unveiled a critical epigenetic signature in MS lesions: oligodendrocytes (OLs) exhibit markedly reduced levels of activating histone mark H3K27ac coupled with elevated levels of repressive histone marks H3K27me3 and H3K9me3 [14]. This pattern creates an epigenetic silencing barrier that prevents myelin production even when mature OLs remain present in lesions [3]. In contrast to developmental myelination, where histone deacetylation enables expression of oligodendrocyte transcriptional profiles, MS involves an apparent shift toward aberrant histone acetylation patterns [15].
ESI1 directly addresses this imbalance by:
- Tripling levels of the activating H3K27ac histone mark [14]
- Sharply reducing levels of repressive histone marks H3K27me3 and H3K9me3 [14]
- Upregulating genes associated with OL maturation and myelin ensheathment [16]
Biomolecular Condensates and Lipid Synthesis for Myelin
Beyond chromatin remodeling, ESI1 operates through a fascinating mechanism involving phase-separated condensates. These specialized membrane-less regulatory hubs within cell nuclei serve as central coordination points for lipid and cholesterol synthesis which are essential components for myelin production [14].
ESI1 treatment induces pronounced formation of nuclear puncta containing SREBP1a or SREBP2, master regulators of lipid metabolism, with liquid-liquid phase-separation-like properties [3]. Additionally, ESI1 promotes concentration of MED1 and RNAPII in these SREBP-containing nuclear puncta [16]. Through these biomolecular condensates, ESI1 drives expression of enzymes involved in lipid and cholesterol biosynthesis, providing the building blocks necessary for myelin sheath formation [3].
Myelin Regeneration in Human Brain Organoids
To evaluate translational potential, researchers tested ESI1 in human embryonic stem cell-derived oligodendrocyte progenitors, as well as in sophisticated human iPSC-derived myelinating organoids [3]. Upon ESI1 treatment, these organoids displayed longer myelin sheaths with improved distribution of sheath lengths at the single-cell level compared to vehicle controls [3]. In parallel experiments with ESI1-treated mice, researchers observed substantial increases in MBP-positive myelinating areas and Plp1-positive mature oligodendrocytes [16], indicating successful remyelination capacity across species.
Clinical Translation and Future of Remyelination Therapies
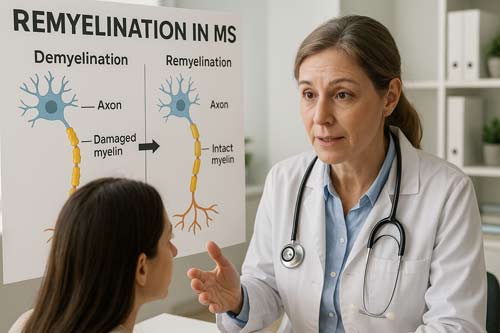
Clinical research in remyelination therapies has accelerated rapidly, with several promising candidates advancing through the development pipeline. These emerging treatments aim to address a critical unmet need in MS management: repairing damaged myelin rather than merely preventing further immune-mediated damage.
PIPE-307 Phase I and II Trial Status
Following successful completion of two Phase I clinical trials that confirmed safety in healthy volunteers, PIPE-307 has progressed to Phase II testing [17]. Currently, Contineum Therapeutics has completed enrollment for the PIPE-307 VISTA trial, recruiting 168 participants across 21 sites in the United States [18][19]. This randomized, double-blind, placebo-controlled study features:
- 26-week treatment duration with two different PIPE-307 doses versus placebo
- Primary endpoints measuring safety and changes in binocular low contrast letter acuity
- Secondary endpoints including MRI measures of myelination, neurofilament light chain, and functional assessments
The final patient is expected to complete the trial by the third quarter of 2025 [17], potentially establishing PIPE-307 as the first approved remyelination therapy for MS patients. Importantly, PIPE-307 is being tested alongside existing disease-modifying treatments rather than as a standalone therapy [2].
Potential of ESI1 in Age-Related Cognitive Decline
Beyond its applications in MS, ESI1 shows promise for treating age-related cognitive impairment. Since myelin loss plays a substantial role in age-related cognitive function deterioration [4], ESI1’s ability to reactivate dormant oligodendrocytes opens new therapeutic avenues. Unlike traditional approaches that focused on oligodendrocyte precursor development, ESI1 demonstrates that reversing epigenetic silencing in mature oligodendrocytes can enable myelin regeneration [4].
At present, researchers are evaluating appropriate dosing strategies, including potential “pulsed therapy” during specific time windows to optimize ESI1’s effects [4]. Therefore, additional studies are needed before human clinical trials can commence.
Combining Remyelination Drugs with Immunotherapies
The future of MS treatment likely lies in combination approaches that simultaneously target different aspects of the disease. Although past combination trials of purely anti-inflammatory agents have yielded disappointing results [20], the pairing of remyelination agents with immunomodulatory drugs shows greater promise [21][20].
This concept is exemplified by an ongoing trial combining metformin with clemastine alongside disease-modifying therapy [22]. Such complementary approaches could potentially:
- Prevent new damage through immunomodulation
- Repair existing damage through remyelination
- Protect axons from progressive degeneration
For optimal outcomes, experts increasingly advocate early intervention with remyelination therapies, as chronically demyelinated axons eventually degenerate beyond the point where remyelination becomes possible [21].

Conclusion
The Promising Horizon of Remyelination Therapies
Remyelination research has reached a remarkable moment in the quest to transform multiple sclerosis (MS) treatment. Emerging therapies like PIPE-307 and ESI1 reflect a major shift from solely targeting inflammation to actively promoting myelin repair—addressing a long-standing gap in MS management.
Traditionally, remyelination failure was thought to result from a loss of oligodendrocyte precursor cells (OPCs). However, recent discoveries reveal that mature oligodendrocytes often persist but are epigenetically silenced or inhibited by specific receptor pathways. This deeper understanding of MS pathophysiology has opened the door to novel therapeutic strategies.
PIPE-307 and ESI1 represent two complementary approaches:
- PIPE-307 selectively blocks the M1 muscarinic receptor (Ki = 4.6 nM), reactivating OPCs and promoting myelination.
- ESI1 restores function in epigenetically silenced mature oligodendrocytes by modifying histones and forming biomolecular condensates.
Both compounds have shown strong efficacy in preclinical models, with PIPE-307 now progressing through Phase II trials. These agents do not aim to replace current disease-modifying therapies (DMTs) but to complement them—offering a multi-modal strategy that combines immunomodulation with direct myelin repair. Early intervention using this dual approach may help preserve axonal integrity and delay irreversible neurodegeneration.
Importantly, the potential of remyelination extends beyond MS. Conditions like age-related cognitive decline, where myelin degradation also plays a role, may benefit from therapies such as ESI1, which target mature oligodendrocytes independent of precursor cell populations.
While further studies are needed to determine optimal dosing, long-term safety, and potential synergy with existing treatments, the progress seen with PIPE-307 is highly encouraging. These developments mark the most significant advance in addressing MS pathology in decades, offering real hope for improved functional outcomes and quality of life for patients.
Frequently Asked Questions:
FAQs
Q1. What is remyelination and why is it important in multiple sclerosis (MS) treatment? Remyelination is the process of repairing damaged myelin, the protective coating around nerve fibers. It’s crucial in MS treatment because it can potentially reverse nerve damage, improve neurological function, and slow disease progression.
Q2. How do PIPE-307 and ESI1 differ in their approach to promoting remyelination? PIPE-307 targets the M1R receptor to activate oligodendrocyte precursor cells, while ESI1 works by reprogramming mature oligodendrocytes through epigenetic modifications. Both aim to enhance myelin production but through different mechanisms.
Q3. Are these new remyelination therapies meant to replace current MS treatments? No, remyelination therapies like PIPE-307 and ESI1 are designed to complement existing immunomodulatory treatments. The goal is to combine approaches that prevent new damage and repair existing damage for more comprehensive MS management.
Q4. What stage of development are these new remyelination therapies in? PIPE-307 has completed Phase I trials and is currently in Phase II clinical trials. ESI1 is still in preclinical stages, showing promise in laboratory and animal studies but not yet tested in human clinical trials.
Q5. Could remyelination therapies have applications beyond MS treatment? Yes, remyelination therapies show potential for treating age-related cognitive decline, which also involves myelin degradation. ESI1, in particular, may have broader applications due to its ability to reactivate dormant oligodendrocytes through epigenetic mechanisms.
References:
[1] – https://pmc.ncbi.nlm.nih.gov/articles/PMC6721708/
[2] – https://www.msaustralia.org.au/news/promising-new-treatment-in-the-pipeline-for-remyelination-in-ms/
[3] – https://www.sciencedirect.com/science/article/abs/pii/S0092867424004008
[4] – https://neurosciencenews.com/myelin-repair-ms-neurology-26027/
[5] – https://link.springer.com/article/10.1007/s00401-020-02189-9
[6] – https://pmc.ncbi.nlm.nih.gov/articles/PMC11317586/
[7] – https://www.researchgate.net/publication/382740212_Targeting_the_
muscarinic_M1_receptor_with_a_selective_brain-penetrant_antagonist_to_promote_remyelination_in_multiple_sclerosis
[8] – https://www.sciencedirect.com/science/article/abs/pii/S0014299904000913
[9] – https://onlinelibrary.wiley.com/doi/full/10.1042/bc20090171
[10] – https://www.pnas.org/doi/10.1073/pnas.2414324121
[11] – https://www.ucsf.edu/news/2024/07/428126/could-new-drug-turn-back-clock-multiple-sclerosis
[12] – https://pubmed.ncbi.nlm.nih.gov/39083422/
[13] – https://www.managedhealthcareexecutive.com/view/potential-first-in-class-m1r-antagonist-may-promote-remyelination-and-restore-function-in-patients-with-ms
[14] – https://scienceblog.cincinnatichildrens.org/small-molecule-shows-early-stage-promise-for-repairing-myelin-sheath-damage/
[15] – https://pmc.ncbi.nlm.nih.gov/articles/PMC6013900/
[16] – https://www.cell.com/cell/abstract/S0092-8674(24)00400-8
[17] – https://ir.contineum-tx.com/news-releases/news-release-details/contineum-therapeutics-completes-enrollment-phase-2-pipe-307
[18] – https://www.clinicaltrialsarena.com/news/contineum-multiple-sclerosis-trial/
[19] – https://multiplesclerosisnewstoday.com/news-posts/2025/01/13/enrollment-complete-phase-2-trial-rrms-remyelination-therapy/
[20] – https://www.tandfonline.com/doi/abs/10.1080/14737175.2023.2289572
[21] – https://pmc.ncbi.nlm.nih.gov/articles/PMC4674562/
[22] – https://www.mssociety.org.uk/research/explore-our-research/search-our-research-projects/can-metformin-clemastine-repair-myelin-people-MS

