CRISPR vs Gene Therapy for Sickle Cell: Which Treatment Works Better?

Introduction
Sickle cell disease (SCD) remains one of the most prevalent inherited hematologic disorders globally, affecting millions of individuals across diverse populations. It is estimated that approximately 100,000 people in the United States live with the condition, with the majority being of African American descent. Worldwide, more than 300,000 infants are diagnosed each year, underscoring its significant global health burden.
In December 2023, the United States Food and Drug Administration (FDA) approved CASGEVY™ (exagamglogene autotemcel, or exa-cel), the first CRISPR-Cas9 gene-edited, cell-based therapy for patients aged 12 years and older with sickle cell disease who experience recurrent vaso-occlusive crises. This historic approval represents a major milestone in molecular medicine and a paradigm shift in the management of SCD. For decades, the therapeutic landscape of sickle cell disease has been limited to symptomatic management and supportive care, including hydroxyurea therapy, blood transfusions, and, in select cases, hematopoietic stem cell transplantation. However, none of these traditional treatments has offered a universally curative solution.
Gene therapy has emerged as a transformative approach because SCD is caused by a single point mutation in the β-globin gene (HBB), resulting in the production of abnormal hemoglobin S. The monogenic nature of the disease makes it an ideal candidate for genetic correction strategies. CRISPR-Cas9 technology, which enables precise editing of targeted genomic sequences, allows for correction of the defective gene or reactivation of fetal hemoglobin (HbF) expression, thereby reducing sickling and associated complications. This mechanism differs fundamentally from earlier gene therapy approaches, which relied on viral vector-mediated insertion of functional β-globin genes rather than direct correction of the genetic defect.
Comparing CRISPR-based interventions with conventional gene therapy raises several important clinical and ethical considerations. While CRISPR offers enhanced precision and potentially durable efficacy, questions remain regarding off-target effects, long-term safety, and scalability. Additionally, the high cost of these treatments and the complex infrastructure required for delivery pose significant barriers to equitable access, particularly in low- and middle-income countries where the disease burden is greatest.
This review explores the underlying mechanisms, clinical trial outcomes, and practical implications of CRISPR and other gene therapy modalities in the management of sickle cell disease. By analyzing emerging data, healthcare professionals can gain a clearer understanding of the therapeutic potential, limitations, and future directions of these groundbreaking technologies. Although early results demonstrate promising curative potential and substantial reductions in vaso-occlusive events, long-term follow-up data are needed to fully assess durability, safety, and cost-effectiveness.
In summary, the approval of CRISPR-Cas9–based therapy for sickle cell disease marks a pivotal advancement in precision medicine. As research continues to evolve, the integration of gene editing into clinical practice will require ongoing collaboration among clinicians, geneticists, policymakers, and global health organizations to ensure that the promise of these therapies translates into accessible, sustainable, and equitable care for all affected individuals.
Keywords: sickle cell disease, CRISPR-Cas9, gene therapy, exagamglogene autotemcel, CASGEVY, hematology, precision medicine
Understanding the Science Behind CRISPR and Gene Therapy
Both gene therapy approaches for sickle cell disease aim to address the underlying genetic mutation, yet they employ fundamentally different mechanisms to achieve this goal. Understanding these distinct scientific approaches helps clinicians evaluate which treatment might best suit individual patients.
CRISPR-Cas9: Targeted DNA Editing via BCL11A Disruption
CRISPR-Cas9 for sickle cell disease works by disrupting the BCL11A gene, which normally functions as a repressor of fetal hemoglobin production after birth [1]. The CRISPR-based treatment CASGEVY (exa-cel) utilizes a ribonucleoprotein complex consisting of Streptococcus pyogenes Cas9 protein and a single guide RNA (gRNA-68) to target and edit specific DNA sequences [1]. This gRNA-68 precisely targets sites 246 base pairs upstream of the transcriptional start in the nearly identical HBG1 and HBG2 genes [1].
The editing process creates multiple outcomes, including an approximately 5-kb intergenic deletion that produces a single hybrid gene with the HBG2 promoter sequence fused to the HBG1 gene [1]. This precise disruption effectively “turns off” the BCL11A repression mechanism, allowing red blood cells to produce fetal hemoglobin again [1]. In clinical studies, this approach achieved 80.5±9.8% on-target editing frequency in healthy donors and 85.8±14.7% in persons with sickle cell disease [1].
Gene Addition Therapy: Lentiviral Vectors and β-globin Transfer
Alternatively, LentiGlobin (lovotibeglogene autotemcel) and LYFGENIA employ lentiviral vectors to add functional genes rather than edit existing ones. These therapies consist of autologous transplantation of hematopoietic stem and progenitor cells transduced with the BB305 lentiviral vector encoding a modified β-globin gene [2]. The vector produces HbAT87Q, a modified adult hemoglobin with an amino acid substitution (threonine to glutamine at position 87) specifically designed to inhibit polymerization of sickle hemoglobin [2].
The lentiviral approach requires careful vector construction since these vectors cannot accommodate the entire β-locus control region (βLCR) essential for regulated expression. Instead, they incorporate a 2.6 to 3.4 kb ‘mini-LCR’ containing extended hypersensitive sites HS2, HS3, and sometimes HS4 [3]. The vector copy number typically ranges from 1.0 to 1.2 copies per cell in transduced patient cells [4].
Fetal Hemoglobin Induction: Shared Goal, Different Paths
Although these technologies operate through distinct mechanisms, they share a common objective—increasing the production of non-sickling hemoglobin to prevent disease manifestations. The CRISPR approach focuses on reactivating natural fetal hemoglobin production by removing genetic repression, whereas gene therapy introduces an engineered β-globin variant that directly inhibits sickling [5].
Following CRISPR editing, patients demonstrated substantial increases in fetal hemoglobin, reaching 19.0 to 26.8% of total hemoglobin with F-cells comprising 69.7 to 87.8% of total red cells [1]. In comparison, the LentiGlobin approach showed median HbAT87Q levels of at least 5.1 g per deciliter, contributing to at least 40% of total hemoglobin, with minimal fetal hemoglobin production [2].
Both treatments require extraction of the patient’s own CD34+ hematopoietic stem cells, which are modified ex vivo and then reinfused as part of a stem cell transplant process [5].
Mechanism of Action: How Each Therapy Works in Sickle Cell Disease
The molecular mechanisms through which these two therapeutic approaches combat sickle cell disease reveal important distinctions in their fundamental strategies, efficacy, and potential long-term outcomes.
CRISPR Sickle Cell Approach: Enhancing HbF via Gene Editing
CRISPR technology treats sickle cell disease by directly modifying the genetic regulation of fetal hemoglobin (HbF). The mechanism centers on disrupting BCL11A, a critical repressor of HbF production. During the process, a CRISPR-Cas9 ribonucleoprotein complex creates precise double-strand DNA breaks at targeted locations within the erythroid-specific enhancer regions of BCL11A. Subsequently, the cell’s error-prone nonhomologous end joining (NHEJ) repair mechanism generates insertions or deletions that effectively disable the gene’s regulatory function [6].
This targeted disruption removes the natural silencing of fetal hemoglobin that typically occurs during development. After successful editing, patients demonstrate substantial HbF induction, with fetal hemoglobin comprising 19.0-26.8% of total hemoglobin [7]. Moreover, the distribution of HbF remains broadly consistent across red blood cells, with F-cells (cells containing fetal hemoglobin) making up 69.7-87.8% of total red cells [7]. This near-pancellular expression pattern closely resembles naturally occurring hereditary persistence of fetal hemoglobin.
Gene Therapy for Sickle Cell Disease: Antisickling Globin Expression
Conversely, lentiviral gene therapy approaches operate by introducing a modified β-globin gene that produces an engineered antisickling protein. The BB305 lentiviral vector delivers a modified β-globin gene that produces HbAT87Q, a hemoglobin variant with a critical amino acid substitution (threonine to glutamine at position 87) [8]. This strategic modification sterically inhibits sickle hemoglobin polymerization under deoxygenated conditions [8].
Clinical data demonstrate that vector-transduced cells maintain stable vector copy numbers of at least 1.1 copies per diploid genome [8]. Additionally, the modified cells produce median HbAT87Q levels of at least 5.1 g per deciliter, contributing to approximately 40% of total hemoglobin without additional blood transfusions [8]. This intervention raises total hemoglobin from baseline values of 8.5 g/dL to 11.0 g/dL or higher [8].
Delivery Methods: Ex Vivo Editing vs Viral Vector Infusion
Both therapeutic approaches utilize ex vivo modification of autologous hematopoietic stem and progenitor cells. For CRISPR therapy, CD34+ cells are collected via apheresis, electroporated with the CRISPR-Cas9 system, and reinfused after myeloablative conditioning with busulfan [9]. The edited cells subsequently engraft in the bone marrow, generating red blood cells with increased fetal hemoglobin [1].
For lentiviral gene therapy, the process begins with stem cell mobilization using plerixafor, followed by apheresis collection [8]. The collected cells undergo transduction with the BB305 lentiviral vector before reinfusion [8]. While both approaches require myeloablative conditioning, the fundamental difference lies in the cellular modification method—direct genome editing versus viral vector-mediated gene addition—resulting in distinct therapeutic proteins and mechanisms of sickling prevention [5].
Clinical Outcomes and Efficacy Data
Recent clinical trials provide compelling evidence for comparing the efficacy of CRISPR and traditional gene therapy approaches in sickle cell disease treatment. These head-to-head comparisons offer valuable insights for clinicians evaluating treatment options.
CTX001 (CRISPR) Trial Results: 93.5% VOC-Free Patients
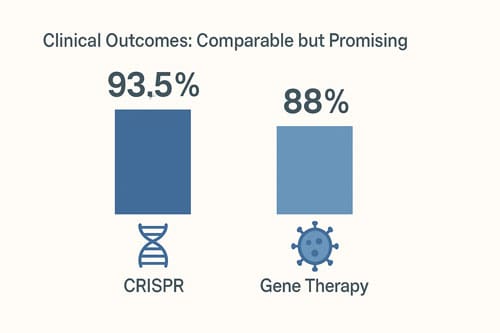
FDA-reviewed data demonstrates remarkable efficacy for CRISPR technology in sickle cell disease. In pivotal trials evaluating CASGEVY (exagamglogene autotemcel), 29 of 31 patients (93.5%) achieved freedom from severe vaso-occlusive crises (VOCs) for at least 12 consecutive months during the 24-month follow-up period [1]. Notably, all seven patients with severe sickle cell disease in another clinical study remained VOC-free with follow-up ranging from five to 22 months after CTX001 infusion [10]. Long-term data confirms this durability, as patients demonstrated sustained responses through extended observation periods.
LentiGlobin and LYFGENIA: Transfusion Independence Rates
In contrast, LYFGENIA (lovotibeglogene autotemcel) showed 88% of treated patients (28 of 32) achieved complete resolution of vaso-occlusive events between 6 and 18 months post-infusion [1]. Furthermore, in the HGB-206 study, all 25 evaluable patients experienced resolution of severe vaso-occlusive events, compared to a median of 3.5 events per year before treatment [8]. Consequently, this represented a substantial clinical improvement for most recipients.
Hemoglobin Levels Post-Treatment: 12g/dL vs 10g/dL
Hemoglobin improvements varied between the two approaches. CTX001-treated patients exhibited total hemoglobin increases from 8.9 to 16.9 g/dL [10], with most achieving levels in the normal range. For instance, the first treated patient saw hemoglobin rise from 7.2 g/dL to 11.8 g/dL nine months after treatment [11]. Similarly, LentiGlobin recipients showed median total hemoglobin increases from baseline 8.5 g/dL to at least 11 g/dL from 6 months through 36 months after infusion [8].
F-cell Expression: 99% in CRISPR vs Variable in Gene Therapy
A crucial distinction exists in cellular distribution of therapeutic proteins. CRISPR-treated patients demonstrated nearly universal expression of fetal hemoglobin, with mean proportion of F-cells (red blood cells expressing HbF) reaching 97.8% (range: 94.6-99.9%) [2]. This near-pancellular distribution likely contributes to CRISPR’s robust clinical efficacy. However, gene therapy approaches showed different expression patterns, with HbAT87Q distributed across approximately 85±8% of red cells [8], still providing sufficient coverage for clinical benefit.
Both approaches have transformed quality of life for patients. In fact, treatment with exagamglogene autotemcel (exa-cel) led to improvements in social impact (+16.5), emotional impact (+8.5), and sleep impact (+5.7) for adults, with similar gains for adolescents [4].
Safety, Risks, and Long-Term Monitoring
Alongside remarkable therapeutic potential, both gene-editing and gene therapy approaches for sickle cell disease present distinct safety profiles requiring careful consideration by clinicians and patients.
Off-Target Effects: CRISPR vs Lentiviral Vectors
The FDA has flagged concerns regarding unintended genomic alterations from CRISPR treatment [3]. Nevertheless, comprehensive safety evaluations using genomewide unbiased identification of double-stranded breaks enabled by sequencing (GUIDE-seq) and high-coverage, hybrid-capture experiments have found no evidence of off-target editing in clinical studies [12]. Lentiviral vectors present different concerns, primarily insertional oncogenesis risk, where vector integration could potentially disrupt normal gene function [13]. This remains a theoretical risk requiring ongoing surveillance.
Conditioning Regimens: Busulfan vs Melphalan
Both therapies require myeloablative conditioning, with busulfan utilized in CRISPR approaches and either busulfan or melphalan in gene therapy protocols. Studies report veno-occlusive liver disease with sinusoidal obstruction syndrome (VOD-SOS) reaching grade 3 severity in some patients, albeit resolving with treatment [12]. According to clinical data, myeloablative conditioning triggers numerous adverse events in 100% of patients, though most are non-serious [13]. Additionally, these regimens carry potential infertility risks, with out-of-pocket fertility preservation costs reaching as high as $40,000 [3].
Adverse Events: VOD, Neutropenia, and Malignancy Risk
Common side effects include low platelet and white blood cell counts, mouth sores (stomatitis), febrile neutropenia, and abdominal pain [1]. Specifically, delayed platelet engraftment affects approximately 4% of LYFGENIA patients, who required more than 100 days post-treatment to achieve recovery [13]. Of particular concern, LYFGENIA carries a black box warning regarding hematologic malignancy risk, with two patients developing acute myeloid leukemia and one diagnosed with myelodysplastic syndrome during clinical development [13].
FDA Mandated 15-Year Follow-Up for Both Therapies
Given these points, the FDA has mandated 15-year follow-up studies for both therapies [5]. This monitoring includes complete blood counts at least every six months and integration site analysis at months 6 and 12 [13]. Regular evaluations are essential as data about longer-term outcomes remain limited, though most treated patients have shown complete resolution of pain crises during available follow-up periods [14].
Cost, Access, and Ethical Considerations
Beyond the impressive clinical outcomes, the financial reality of gene-based treatments presents substantial barriers. CASGEVY costs $2.2 million per treatment while LYFGENIA comes with an even steeper price tag of $3.1 million [15][16]. Economic analyzes suggest that prices below $2 million would be considered cost-effective from a societal perspective [17][18], indicating current prices exceed optimal value-based thresholds.
Insurance coverage remains fragmented across payer types. Primarily, between 50-60% of individuals with sickle cell disease rely on Medicaid [19][16], yet state Medicaid programs vary in their participation in the Centers for Medicare & Medicaid Services Cell and Gene Therapy Access Model [20]. Medicare has approved new technology add-on payments of maximum $2,325,000 for LYFGENIA and $1,650,000 for CASGEVY in fiscal year 2025 [14]. Meanwhile, private insurers often impose stricter eligibility criteria than FDA guidelines [14].
Essentially, ethical concerns extend into both clinical and societal domains. Currently, the disproportionate SCD burden in Black communities coupled with median Black household incomes of $54,000 [15] creates profound equity challenges. Furthermore, innovative payment models under consideration include warranty models, where manufacturers would reimburse payers if treatment fails, and outcome-based arrangements linking payment to clinical results [14].
The global access picture appears even more concerning. Many low-and-middle-income countries (LMICs) face insurmountable barriers despite carrying nearly 90% of the global disease burden [21]. Value-based prices range from $3.6 million in the US to merely $700 in Uganda [22]. Therefore, manufacturing costs, limited research capacity, and inadequate technology infrastructure in LMICs collectively threaten equitable worldwide distribution of these potentially curative therapies [22].
Comparison Table
| Comparison Factor | CRISPR (CASGEVY) | Gene Therapy (LYFGENIA/LentiGlobin) |
| Mechanism of Action | Disrupts BCL11A gene to reactivate fetal hemoglobin production | Uses lentiviral vectors to add modified β-globin gene (HbAT87Q) |
| Treatment Process | Ex vivo modification of CD34+ cells via electroporation | Ex vivo modification of CD34+ cells via viral transduction |
| Clinical Efficacy | 93.5% (29/31) patients VOC-free for 12+ months | 88% (28/32) patients achieved VOC resolution |
| Hemoglobin Improvement | 8.9 to 16.9 g/dL | 8.5 to 11.0 g/dL |
| Therapeutic Protein Distribution | 97.8% F-cells (range: 94.6-99.9%) | ~85±8% of red cells |
| Cost | $2.2 million per treatment | $3.1 million per treatment |
| Primary Safety Concerns | Potential off-target genomic alterations | Risk of insertional oncogenesis; Black box warning for hematologic malignancy |
| Conditioning Agent | Busulfan | Busulfan or Melphalan |
| FDA Age Requirement | ≥12 years | ≥12 years |
| Required Follow-up | 15 years | 15 years |
Conclusion 
Both CRISPR and traditional gene therapy approaches represent unprecedented advancements in sickle cell disease treatment. These revolutionary technologies address the fundamental genetic defect underlying this debilitating condition, though they employ markedly different mechanisms to achieve similar clinical outcomes. CASGEVY utilizes precise genetic editing to reactivate natural fetal hemoglobin production, whereas LYFGENIA and related therapies introduce modified antisickling β-globin through lentiviral vectors. The clinical results from recent trials demonstrate remarkable efficacy for both approaches, with CRISPR showing marginally superior VOC resolution rates (93.5% versus 88%) and hemoglobin improvement profiles.
Despite these impressive clinical outcomes, several challenges remain unresolved. Safety concerns, albeit different for each approach, require vigilant monitoring. CRISPR technology carries theoretical risks of off-target genetic modifications, while gene therapy approaches face potential insertional oncogenesis hazards—evidenced by LYFGENIA’s black box warning for hematologic malignancy risk. Additionally, both treatments necessitate myeloablative conditioning regimens that introduce substantial short-term toxicity and potential long-term fertility implications.
Perhaps the most formidable barrier to widespread implementation stems from economic considerations rather than scientific limitations. The extraordinary price tags—$2.2 million for CASGEVY and $3.1 million for LYFGENIA—place these potentially curative therapies beyond reach for many patients, particularly those in low-and-middle-income countries where disease burden disproportionately concentrates. These financial hurdles, coupled with complex infrastructure requirements, threaten to worsen existing healthcare disparities unless innovative payment models and accessibility initiatives emerge.
Furthermore, long-term durability remains somewhat uncertain. Though current data suggest sustained therapeutic effects, the mandated 15-year follow-up studies will prove essential to fully characterize these treatments’ longevity and late-onset complications. Healthcare practitioners must therefore balance enthusiasm for these groundbreaking technologies against pragmatic recognition of their limitations.
The question “which treatment works better?” lacks a straightforward answer. Each approach offers distinct advantages—CRISPR provides near-pancellular protein distribution and potentially superior hemoglobin improvements, while gene therapy boasts extensive clinical experience and well-characterized vector behavior. Patient-specific factors including age, disease severity, comorbidities, and healthcare access will undoubtedly influence individual treatment selection. Healthcare providers must therefore carefully weigh these considerations alongside evolving clinical data to make personalized recommendations.
These revolutionary therapies nevertheless mark a historic turning point in sickle cell disease management. After decades of limited treatment options, affected individuals now have potential access to functional cures. The path forward requires continued scientific innovation coupled with determined efforts to enhance affordability and accessibility. Otherwise, these remarkable technological achievements risk becoming marvels admired but unreachable by those who need them most.
Key Takeaways
Both CRISPR and gene therapy offer groundbreaking approaches to treating sickle cell disease, each with distinct mechanisms and outcomes that healthcare providers must carefully consider.
- CRISPR shows superior clinical outcomes: 93.5% of patients achieved VOC-free status versus 88% with gene therapy, plus higher hemoglobin levels (16.9 vs 11.0 g/dL)
- Cost remains a major barrier: CRISPR costs $2.2M while gene therapy costs $3.1M per treatment, creating significant access challenges for most patients
- Different safety profiles require monitoring: CRISPR risks off-target genetic effects while gene therapy carries insertional oncogenesis risk and hematologic malignancy warnings
- Both require intensive treatment protocols: Patients need myeloablative conditioning, 15-year follow-up monitoring, and face potential fertility complications
- Global equity concerns persist: High costs and infrastructure requirements threaten to worsen healthcare disparities, especially in low-income countries where disease burden is highest
The choice between treatments depends on individual patient factors, insurance coverage, and access to specialized centers, rather than a clear “winner” in effectiveness.
Frequently Asked Questions:
FAQs
Q1. What are the latest treatment options for sickle cell disease in 2025? The FDA has recently approved two groundbreaking treatments for sickle cell disease: CASGEVY, a CRISPR-based gene-editing therapy, and LYFGENIA, a gene therapy using lentiviral vectors. Both aim to address the underlying genetic cause of the disease and have shown promising results in clinical trials.
Q2. How do CRISPR and gene therapy differ in treating sickle cell disease? CRISPR technology (CASGEVY) works by editing genes to reactivate fetal hemoglobin production, while gene therapy (LYFGENIA) introduces a modified β-globin gene to produce anti-sickling hemoglobin. Both approaches have shown high efficacy in reducing vaso-occlusive crises, but they differ in their mechanisms and potential side effects.
Q3. What are the success rates of these new sickle cell treatments? Clinical trials have shown impressive results. CASGEVY (CRISPR) demonstrated 93.5% of patients remaining free from severe vaso-occlusive crises for at least 12 months, while LYFGENIA (gene therapy) showed 88% of patients achieving complete resolution of vaso-occlusive events. Both treatments significantly improved patients’ hemoglobin levels and quality of life.
Q4. How much do these new sickle cell treatments cost? The cost of these treatments is substantial. CASGEVY is priced at $2.2 million per treatment, while LYFGENIA costs $3.1 million. These high prices pose significant challenges for accessibility and may require innovative payment models to ensure patients can benefit from these potentially curative therapies.
Q5. What are the long-term safety considerations for these new treatments? Both treatments require careful long-term monitoring. The FDA has mandated 15-year follow-up studies to assess potential long-term effects. CRISPR therapy carries theoretical risks of off-target genetic modifications, while gene therapy has a black box warning for hematologic malignancy risk. Additionally, both treatments involve myeloablative conditioning, which can have short-term toxicity and potential long-term fertility implications.
References:
[1] – https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapies-treat-patients-sickle-cell-disease
[2] – https://ir.crisprtx.com/static-files/1db0ff23-41dd-4f1a-a523-456ecf7991b8
[3] – https://www.theguardian.com/us-news/2023/dec/08/fda-new-treatment-sickle-cell-disease
[4] – https://www.hematology.org/newsroom/press-releases/2025/gene-therapy-leads-to-improved-quality-of-life
[5] – https://patienteducation.asgct.org/disease-treatments/sickle-cell-disease
[6] – https://pmc.ncbi.nlm.nih.gov/articles/PMC8526756/
[7] – https://www.nejm.org/doi/full/10.1056/NEJMoa2215643
[8] – https://www.nejm.org/doi/full/10.1056/NEJMoa2117175
[9] – https://pmc.ncbi.nlm.nih.gov/articles/PMC9069474/
[10] – https://ir.crisprtx.com/news-releases/news-release-details/vertex-and-crispr-therapeutics-present-new-data-22-patients
[11] – https://sicklecellanemianews.com/news/first-scd-patient-treated-with-ctx001-remains-vocs-free-after-9-months-phase-1-2-trialshows/
[12] – https://www.nejm.org/doi/full/10.1056/NEJMoa2031054
[13] – https://www.fda.gov/media/174610/download
[14] – https://www.cbo.gov/publication/61149
[15] – https://theblackwallsttimes.com/2025/03/18/sickle-cell-treatment-that-cured-new-york-patient-costs-millions/
[16] – https://www.senatedems.ct.gov/senate-passes-legislation-to-expand-coverage-for-sickle-cell-disease
[17] – https://pubmed.ncbi.nlm.nih.gov/38252942/
[18] – https://www.ajmc.com/view/analysis-explores-gene-therapy-s-potential-to-be-cost-effective-in-scd
[19] – https://www.childrenshospitals.org/news/cha-blog/2025/01/increasing-access-to-revolutionary-sickle-cell-therapies
[20] – https://stateline.org/2024/03/14/new-way-for-states-to-cover-pricey-gene-therapies-will-start-with-sickle-cell-disease/
[21] – https://www3.weforum.org/docs/WEF_Accelerating_Global_Access_to_
Gene_Therapies_2022.pdf
[22] – https://pmc.ncbi.nlm.nih.gov/articles/PMC10834512/
CRISPR vs Gene Therapy for Sickle Cell: Which Treatment Works Better?

Introduction
Sickle cell disease (SCD) remains one of the most prevalent inherited hematologic disorders globally, affecting millions of individuals across diverse populations. It is estimated that approximately 100,000 people in the United States live with the condition, with the majority being of African American descent. Worldwide, more than 300,000 infants are diagnosed each year, underscoring its significant global health burden.
In December 2023, the United States Food and Drug Administration (FDA) approved CASGEVY™ (exagamglogene autotemcel, or exa-cel), the first CRISPR-Cas9 gene-edited, cell-based therapy for patients aged 12 years and older with sickle cell disease who experience recurrent vaso-occlusive crises. This historic approval represents a major milestone in molecular medicine and a paradigm shift in the management of SCD. For decades, the therapeutic landscape of sickle cell disease has been limited to symptomatic management and supportive care, including hydroxyurea therapy, blood transfusions, and, in select cases, hematopoietic stem cell transplantation. However, none of these traditional treatments has offered a universally curative solution.
Gene therapy has emerged as a transformative approach because SCD is caused by a single point mutation in the β-globin gene (HBB), resulting in the production of abnormal hemoglobin S. The monogenic nature of the disease makes it an ideal candidate for genetic correction strategies. CRISPR-Cas9 technology, which enables precise editing of targeted genomic sequences, allows for correction of the defective gene or reactivation of fetal hemoglobin (HbF) expression, thereby reducing sickling and associated complications. This mechanism differs fundamentally from earlier gene therapy approaches, which relied on viral vector-mediated insertion of functional β-globin genes rather than direct correction of the genetic defect.
Comparing CRISPR-based interventions with conventional gene therapy raises several important clinical and ethical considerations. While CRISPR offers enhanced precision and potentially durable efficacy, questions remain regarding off-target effects, long-term safety, and scalability. Additionally, the high cost of these treatments and the complex infrastructure required for delivery pose significant barriers to equitable access, particularly in low- and middle-income countries where the disease burden is greatest.
This review explores the underlying mechanisms, clinical trial outcomes, and practical implications of CRISPR and other gene therapy modalities in the management of sickle cell disease. By analyzing emerging data, healthcare professionals can gain a clearer understanding of the therapeutic potential, limitations, and future directions of these groundbreaking technologies. Although early results demonstrate promising curative potential and substantial reductions in vaso-occlusive events, long-term follow-up data are needed to fully assess durability, safety, and cost-effectiveness.
In summary, the approval of CRISPR-Cas9–based therapy for sickle cell disease marks a pivotal advancement in precision medicine. As research continues to evolve, the integration of gene editing into clinical practice will require ongoing collaboration among clinicians, geneticists, policymakers, and global health organizations to ensure that the promise of these therapies translates into accessible, sustainable, and equitable care for all affected individuals.
Keywords: sickle cell disease, CRISPR-Cas9, gene therapy, exagamglogene autotemcel, CASGEVY, hematology, precision medicine
Understanding the Science Behind CRISPR and Gene Therapy
Both gene therapy approaches for sickle cell disease aim to address the underlying genetic mutation, yet they employ fundamentally different mechanisms to achieve this goal. Understanding these distinct scientific approaches helps clinicians evaluate which treatment might best suit individual patients.
CRISPR-Cas9: Targeted DNA Editing via BCL11A Disruption
CRISPR-Cas9 for sickle cell disease works by disrupting the BCL11A gene, which normally functions as a repressor of fetal hemoglobin production after birth [1]. The CRISPR-based treatment CASGEVY (exa-cel) utilizes a ribonucleoprotein complex consisting of Streptococcus pyogenes Cas9 protein and a single guide RNA (gRNA-68) to target and edit specific DNA sequences [1]. This gRNA-68 precisely targets sites 246 base pairs upstream of the transcriptional start in the nearly identical HBG1 and HBG2 genes [1].
The editing process creates multiple outcomes, including an approximately 5-kb intergenic deletion that produces a single hybrid gene with the HBG2 promoter sequence fused to the HBG1 gene [1]. This precise disruption effectively “turns off” the BCL11A repression mechanism, allowing red blood cells to produce fetal hemoglobin again [1]. In clinical studies, this approach achieved 80.5±9.8% on-target editing frequency in healthy donors and 85.8±14.7% in persons with sickle cell disease [1].
Gene Addition Therapy: Lentiviral Vectors and β-globin Transfer
Alternatively, LentiGlobin (lovotibeglogene autotemcel) and LYFGENIA employ lentiviral vectors to add functional genes rather than edit existing ones. These therapies consist of autologous transplantation of hematopoietic stem and progenitor cells transduced with the BB305 lentiviral vector encoding a modified β-globin gene [2]. The vector produces HbAT87Q, a modified adult hemoglobin with an amino acid substitution (threonine to glutamine at position 87) specifically designed to inhibit polymerization of sickle hemoglobin [2].
The lentiviral approach requires careful vector construction since these vectors cannot accommodate the entire β-locus control region (βLCR) essential for regulated expression. Instead, they incorporate a 2.6 to 3.4 kb ‘mini-LCR’ containing extended hypersensitive sites HS2, HS3, and sometimes HS4 [3]. The vector copy number typically ranges from 1.0 to 1.2 copies per cell in transduced patient cells [4].
Fetal Hemoglobin Induction: Shared Goal, Different Paths
Although these technologies operate through distinct mechanisms, they share a common objective—increasing the production of non-sickling hemoglobin to prevent disease manifestations. The CRISPR approach focuses on reactivating natural fetal hemoglobin production by removing genetic repression, whereas gene therapy introduces an engineered β-globin variant that directly inhibits sickling [5].
Following CRISPR editing, patients demonstrated substantial increases in fetal hemoglobin, reaching 19.0 to 26.8% of total hemoglobin with F-cells comprising 69.7 to 87.8% of total red cells [1]. In comparison, the LentiGlobin approach showed median HbAT87Q levels of at least 5.1 g per deciliter, contributing to at least 40% of total hemoglobin, with minimal fetal hemoglobin production [2].
Both treatments require extraction of the patient’s own CD34+ hematopoietic stem cells, which are modified ex vivo and then reinfused as part of a stem cell transplant process [5].
Mechanism of Action: How Each Therapy Works in Sickle Cell Disease
The molecular mechanisms through which these two therapeutic approaches combat sickle cell disease reveal important distinctions in their fundamental strategies, efficacy, and potential long-term outcomes.
CRISPR Sickle Cell Approach: Enhancing HbF via Gene Editing
CRISPR technology treats sickle cell disease by directly modifying the genetic regulation of fetal hemoglobin (HbF). The mechanism centers on disrupting BCL11A, a critical repressor of HbF production. During the process, a CRISPR-Cas9 ribonucleoprotein complex creates precise double-strand DNA breaks at targeted locations within the erythroid-specific enhancer regions of BCL11A. Subsequently, the cell’s error-prone nonhomologous end joining (NHEJ) repair mechanism generates insertions or deletions that effectively disable the gene’s regulatory function [6].
This targeted disruption removes the natural silencing of fetal hemoglobin that typically occurs during development. After successful editing, patients demonstrate substantial HbF induction, with fetal hemoglobin comprising 19.0-26.8% of total hemoglobin [7]. Moreover, the distribution of HbF remains broadly consistent across red blood cells, with F-cells (cells containing fetal hemoglobin) making up 69.7-87.8% of total red cells [7]. This near-pancellular expression pattern closely resembles naturally occurring hereditary persistence of fetal hemoglobin.
Gene Therapy for Sickle Cell Disease: Antisickling Globin Expression
Conversely, lentiviral gene therapy approaches operate by introducing a modified β-globin gene that produces an engineered antisickling protein. The BB305 lentiviral vector delivers a modified β-globin gene that produces HbAT87Q, a hemoglobin variant with a critical amino acid substitution (threonine to glutamine at position 87) [8]. This strategic modification sterically inhibits sickle hemoglobin polymerization under deoxygenated conditions [8].
Clinical data demonstrate that vector-transduced cells maintain stable vector copy numbers of at least 1.1 copies per diploid genome [8]. Additionally, the modified cells produce median HbAT87Q levels of at least 5.1 g per deciliter, contributing to approximately 40% of total hemoglobin without additional blood transfusions [8]. This intervention raises total hemoglobin from baseline values of 8.5 g/dL to 11.0 g/dL or higher [8].
Delivery Methods: Ex Vivo Editing vs Viral Vector Infusion
Both therapeutic approaches utilize ex vivo modification of autologous hematopoietic stem and progenitor cells. For CRISPR therapy, CD34+ cells are collected via apheresis, electroporated with the CRISPR-Cas9 system, and reinfused after myeloablative conditioning with busulfan [9]. The edited cells subsequently engraft in the bone marrow, generating red blood cells with increased fetal hemoglobin [1].
For lentiviral gene therapy, the process begins with stem cell mobilization using plerixafor, followed by apheresis collection [8]. The collected cells undergo transduction with the BB305 lentiviral vector before reinfusion [8]. While both approaches require myeloablative conditioning, the fundamental difference lies in the cellular modification method—direct genome editing versus viral vector-mediated gene addition—resulting in distinct therapeutic proteins and mechanisms of sickling prevention [5].
Clinical Outcomes and Efficacy Data
Recent clinical trials provide compelling evidence for comparing the efficacy of CRISPR and traditional gene therapy approaches in sickle cell disease treatment. These head-to-head comparisons offer valuable insights for clinicians evaluating treatment options.
CTX001 (CRISPR) Trial Results: 93.5% VOC-Free Patients
FDA-reviewed data demonstrates remarkable efficacy for CRISPR technology in sickle cell disease. In pivotal trials evaluating CASGEVY (exagamglogene autotemcel), 29 of 31 patients (93.5%) achieved freedom from severe vaso-occlusive crises (VOCs) for at least 12 consecutive months during the 24-month follow-up period [1]. Notably, all seven patients with severe sickle cell disease in another clinical study remained VOC-free with follow-up ranging from five to 22 months after CTX001 infusion [10]. Long-term data confirms this durability, as patients demonstrated sustained responses through extended observation periods.
LentiGlobin and LYFGENIA: Transfusion Independence Rates
In contrast, LYFGENIA (lovotibeglogene autotemcel) showed 88% of treated patients (28 of 32) achieved complete resolution of vaso-occlusive events between 6 and 18 months post-infusion [1]. Furthermore, in the HGB-206 study, all 25 evaluable patients experienced resolution of severe vaso-occlusive events, compared to a median of 3.5 events per year before treatment [8]. Consequently, this represented a substantial clinical improvement for most recipients.
Hemoglobin Levels Post-Treatment: 12g/dL vs 10g/dL
Hemoglobin improvements varied between the two approaches. CTX001-treated patients exhibited total hemoglobin increases from 8.9 to 16.9 g/dL [10], with most achieving levels in the normal range. For instance, the first treated patient saw hemoglobin rise from 7.2 g/dL to 11.8 g/dL nine months after treatment [11]. Similarly, LentiGlobin recipients showed median total hemoglobin increases from baseline 8.5 g/dL to at least 11 g/dL from 6 months through 36 months after infusion [8].
F-cell Expression: 99% in CRISPR vs Variable in Gene Therapy
A crucial distinction exists in cellular distribution of therapeutic proteins. CRISPR-treated patients demonstrated nearly universal expression of fetal hemoglobin, with mean proportion of F-cells (red blood cells expressing HbF) reaching 97.8% (range: 94.6-99.9%) [2]. This near-pancellular distribution likely contributes to CRISPR’s robust clinical efficacy. However, gene therapy approaches showed different expression patterns, with HbAT87Q distributed across approximately 85±8% of red cells [8], still providing sufficient coverage for clinical benefit.
Both approaches have transformed quality of life for patients. In fact, treatment with exagamglogene autotemcel (exa-cel) led to improvements in social impact (+16.5), emotional impact (+8.5), and sleep impact (+5.7) for adults, with similar gains for adolescents [4].

Safety, Risks, and Long-Term Monitoring
Alongside remarkable therapeutic potential, both gene-editing and gene therapy approaches for sickle cell disease present distinct safety profiles requiring careful consideration by clinicians and patients.
Off-Target Effects: CRISPR vs Lentiviral Vectors
The FDA has flagged concerns regarding unintended genomic alterations from CRISPR treatment [3]. Nevertheless, comprehensive safety evaluations using genomewide unbiased identification of double-stranded breaks enabled by sequencing (GUIDE-seq) and high-coverage, hybrid-capture experiments have found no evidence of off-target editing in clinical studies [12]. Lentiviral vectors present different concerns, primarily insertional oncogenesis risk, where vector integration could potentially disrupt normal gene function [13]. This remains a theoretical risk requiring ongoing surveillance.
Conditioning Regimens: Busulfan vs Melphalan
Both therapies require myeloablative conditioning, with busulfan utilized in CRISPR approaches and either busulfan or melphalan in gene therapy protocols. Studies report veno-occlusive liver disease with sinusoidal obstruction syndrome (VOD-SOS) reaching grade 3 severity in some patients, albeit resolving with treatment [12]. According to clinical data, myeloablative conditioning triggers numerous adverse events in 100% of patients, though most are non-serious [13]. Additionally, these regimens carry potential infertility risks, with out-of-pocket fertility preservation costs reaching as high as $40,000 [3].
Adverse Events: VOD, Neutropenia, and Malignancy Risk
Common side effects include low platelet and white blood cell counts, mouth sores (stomatitis), febrile neutropenia, and abdominal pain [1]. Specifically, delayed platelet engraftment affects approximately 4% of LYFGENIA patients, who required more than 100 days post-treatment to achieve recovery [13]. Of particular concern, LYFGENIA carries a black box warning regarding hematologic malignancy risk, with two patients developing acute myeloid leukemia and one diagnosed with myelodysplastic syndrome during clinical development [13].
FDA Mandated 15-Year Follow-Up for Both Therapies
Given these points, the FDA has mandated 15-year follow-up studies for both therapies [5]. This monitoring includes complete blood counts at least every six months and integration site analysis at months 6 and 12 [13]. Regular evaluations are essential as data about longer-term outcomes remain limited, though most treated patients have shown complete resolution of pain crises during available follow-up periods [14].
Cost, Access, and Ethical Considerations
Beyond the impressive clinical outcomes, the financial reality of gene-based treatments presents substantial barriers. CASGEVY costs $2.2 million per treatment while LYFGENIA comes with an even steeper price tag of $3.1 million [15][16]. Economic analyzes suggest that prices below $2 million would be considered cost-effective from a societal perspective [17][18], indicating current prices exceed optimal value-based thresholds.
Insurance coverage remains fragmented across payer types. Primarily, between 50-60% of individuals with sickle cell disease rely on Medicaid [19][16], yet state Medicaid programs vary in their participation in the Centers for Medicare & Medicaid Services Cell and Gene Therapy Access Model [20]. Medicare has approved new technology add-on payments of maximum $2,325,000 for LYFGENIA and $1,650,000 for CASGEVY in fiscal year 2025 [14]. Meanwhile, private insurers often impose stricter eligibility criteria than FDA guidelines [14].
Essentially, ethical concerns extend into both clinical and societal domains. Currently, the disproportionate SCD burden in Black communities coupled with median Black household incomes of $54,000 [15] creates profound equity challenges. Furthermore, innovative payment models under consideration include warranty models, where manufacturers would reimburse payers if treatment fails, and outcome-based arrangements linking payment to clinical results [14].
The global access picture appears even more concerning. Many low-and-middle-income countries (LMICs) face insurmountable barriers despite carrying nearly 90% of the global disease burden [21]. Value-based prices range from $3.6 million in the US to merely $700 in Uganda [22]. Therefore, manufacturing costs, limited research capacity, and inadequate technology infrastructure in LMICs collectively threaten equitable worldwide distribution of these potentially curative therapies [22].
Comparison Table
| Comparison Factor | CRISPR (CASGEVY) | Gene Therapy (LYFGENIA/LentiGlobin) |
| Mechanism of Action | Disrupts BCL11A gene to reactivate fetal hemoglobin production | Uses lentiviral vectors to add modified β-globin gene (HbAT87Q) |
| Treatment Process | Ex vivo modification of CD34+ cells via electroporation | Ex vivo modification of CD34+ cells via viral transduction |
| Clinical Efficacy | 93.5% (29/31) patients VOC-free for 12+ months | 88% (28/32) patients achieved VOC resolution |
| Hemoglobin Improvement | 8.9 to 16.9 g/dL | 8.5 to 11.0 g/dL |
| Therapeutic Protein Distribution | 97.8% F-cells (range: 94.6-99.9%) | ~85±8% of red cells |
| Cost | $2.2 million per treatment | $3.1 million per treatment |
| Primary Safety Concerns | Potential off-target genomic alterations | Risk of insertional oncogenesis; Black box warning for hematologic malignancy |
| Conditioning Agent | Busulfan | Busulfan or Melphalan |
| FDA Age Requirement | ≥12 years | ≥12 years |
| Required Follow-up | 15 years | 15 years |
Conclusion 
Both CRISPR and traditional gene therapy approaches represent unprecedented advancements in sickle cell disease treatment. These revolutionary technologies address the fundamental genetic defect underlying this debilitating condition, though they employ markedly different mechanisms to achieve similar clinical outcomes. CASGEVY utilizes precise genetic editing to reactivate natural fetal hemoglobin production, whereas LYFGENIA and related therapies introduce modified antisickling β-globin through lentiviral vectors. The clinical results from recent trials demonstrate remarkable efficacy for both approaches, with CRISPR showing marginally superior VOC resolution rates (93.5% versus 88%) and hemoglobin improvement profiles.
Despite these impressive clinical outcomes, several challenges remain unresolved. Safety concerns, albeit different for each approach, require vigilant monitoring. CRISPR technology carries theoretical risks of off-target genetic modifications, while gene therapy approaches face potential insertional oncogenesis hazards—evidenced by LYFGENIA’s black box warning for hematologic malignancy risk. Additionally, both treatments necessitate myeloablative conditioning regimens that introduce substantial short-term toxicity and potential long-term fertility implications.
Perhaps the most formidable barrier to widespread implementation stems from economic considerations rather than scientific limitations. The extraordinary price tags—$2.2 million for CASGEVY and $3.1 million for LYFGENIA—place these potentially curative therapies beyond reach for many patients, particularly those in low-and-middle-income countries where disease burden disproportionately concentrates. These financial hurdles, coupled with complex infrastructure requirements, threaten to worsen existing healthcare disparities unless innovative payment models and accessibility initiatives emerge.
Furthermore, long-term durability remains somewhat uncertain. Though current data suggest sustained therapeutic effects, the mandated 15-year follow-up studies will prove essential to fully characterize these treatments’ longevity and late-onset complications. Healthcare practitioners must therefore balance enthusiasm for these groundbreaking technologies against pragmatic recognition of their limitations.
The question “which treatment works better?” lacks a straightforward answer. Each approach offers distinct advantages—CRISPR provides near-pancellular protein distribution and potentially superior hemoglobin improvements, while gene therapy boasts extensive clinical experience and well-characterized vector behavior. Patient-specific factors including age, disease severity, comorbidities, and healthcare access will undoubtedly influence individual treatment selection. Healthcare providers must therefore carefully weigh these considerations alongside evolving clinical data to make personalized recommendations.
These revolutionary therapies nevertheless mark a historic turning point in sickle cell disease management. After decades of limited treatment options, affected individuals now have potential access to functional cures. The path forward requires continued scientific innovation coupled with determined efforts to enhance affordability and accessibility. Otherwise, these remarkable technological achievements risk becoming marvels admired but unreachable by those who need them most.
Key Takeaways
Both CRISPR and gene therapy offer groundbreaking approaches to treating sickle cell disease, each with distinct mechanisms and outcomes that healthcare providers must carefully consider.
- CRISPR shows superior clinical outcomes: 93.5% of patients achieved VOC-free status versus 88% with gene therapy, plus higher hemoglobin levels (16.9 vs 11.0 g/dL)
- Cost remains a major barrier: CRISPR costs $2.2M while gene therapy costs $3.1M per treatment, creating significant access challenges for most patients
- Different safety profiles require monitoring: CRISPR risks off-target genetic effects while gene therapy carries insertional oncogenesis risk and hematologic malignancy warnings
- Both require intensive treatment protocols: Patients need myeloablative conditioning, 15-year follow-up monitoring, and face potential fertility complications
- Global equity concerns persist: High costs and infrastructure requirements threaten to worsen healthcare disparities, especially in low-income countries where disease burden is highest
The choice between treatments depends on individual patient factors, insurance coverage, and access to specialized centers, rather than a clear “winner” in effectiveness.

Frequently Asked Questions:
FAQs
Q1. What are the latest treatment options for sickle cell disease in 2025? The FDA has recently approved two groundbreaking treatments for sickle cell disease: CASGEVY, a CRISPR-based gene-editing therapy, and LYFGENIA, a gene therapy using lentiviral vectors. Both aim to address the underlying genetic cause of the disease and have shown promising results in clinical trials.
Q2. How do CRISPR and gene therapy differ in treating sickle cell disease? CRISPR technology (CASGEVY) works by editing genes to reactivate fetal hemoglobin production, while gene therapy (LYFGENIA) introduces a modified β-globin gene to produce anti-sickling hemoglobin. Both approaches have shown high efficacy in reducing vaso-occlusive crises, but they differ in their mechanisms and potential side effects.
Q3. What are the success rates of these new sickle cell treatments? Clinical trials have shown impressive results. CASGEVY (CRISPR) demonstrated 93.5% of patients remaining free from severe vaso-occlusive crises for at least 12 months, while LYFGENIA (gene therapy) showed 88% of patients achieving complete resolution of vaso-occlusive events. Both treatments significantly improved patients’ hemoglobin levels and quality of life.
Q4. How much do these new sickle cell treatments cost? The cost of these treatments is substantial. CASGEVY is priced at $2.2 million per treatment, while LYFGENIA costs $3.1 million. These high prices pose significant challenges for accessibility and may require innovative payment models to ensure patients can benefit from these potentially curative therapies.
Q5. What are the long-term safety considerations for these new treatments? Both treatments require careful long-term monitoring. The FDA has mandated 15-year follow-up studies to assess potential long-term effects. CRISPR therapy carries theoretical risks of off-target genetic modifications, while gene therapy has a black box warning for hematologic malignancy risk. Additionally, both treatments involve myeloablative conditioning, which can have short-term toxicity and potential long-term fertility implications.
References:
[1] – https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapies-treat-patients-sickle-cell-disease
[2] – https://ir.crisprtx.com/static-files/1db0ff23-41dd-4f1a-a523-456ecf7991b8
[3] – https://www.theguardian.com/us-news/2023/dec/08/fda-new-treatment-sickle-cell-disease
[4] – https://www.hematology.org/newsroom/press-releases/2025/gene-therapy-leads-to-improved-quality-of-life
[5] – https://patienteducation.asgct.org/disease-treatments/sickle-cell-disease
[6] – https://pmc.ncbi.nlm.nih.gov/articles/PMC8526756/
[7] – https://www.nejm.org/doi/full/10.1056/NEJMoa2215643
[8] – https://www.nejm.org/doi/full/10.1056/NEJMoa2117175
[9] – https://pmc.ncbi.nlm.nih.gov/articles/PMC9069474/
[10] – https://ir.crisprtx.com/news-releases/news-release-details/vertex-and-crispr-therapeutics-present-new-data-22-patients
[11] – https://sicklecellanemianews.com/news/first-scd-patient-treated-with-ctx001-remains-vocs-free-after-9-months-phase-1-2-trialshows/
[12] – https://www.nejm.org/doi/full/10.1056/NEJMoa2031054
[13] – https://www.fda.gov/media/174610/download
[14] – https://www.cbo.gov/publication/61149
[15] – https://theblackwallsttimes.com/2025/03/18/sickle-cell-treatment-that-cured-new-york-patient-costs-millions/
[16] – https://www.senatedems.ct.gov/senate-passes-legislation-to-expand-coverage-for-sickle-cell-disease
[17] – https://pubmed.ncbi.nlm.nih.gov/38252942/
[18] – https://www.ajmc.com/view/analysis-explores-gene-therapy-s-potential-to-be-cost-effective-in-scd
[19] – https://www.childrenshospitals.org/news/cha-blog/2025/01/increasing-access-to-revolutionary-sickle-cell-therapies
[20] – https://stateline.org/2024/03/14/new-way-for-states-to-cover-pricey-gene-therapies-will-start-with-sickle-cell-disease/
[21] – https://www3.weforum.org/docs/WEF_Accelerating_Global_Access_to_
Gene_Therapies_2022.pdf
[22] – https://pmc.ncbi.nlm.nih.gov/articles/PMC10834512/

