Iron Study Interpretation: Why Traditional Tests Miss Functional Deficiency
Introduction
Anemia is one of the most common diseases in the world, and iron deficiency anemia is the most common type, according to several studies. Understanding iron studies correctly is still challenging but essential for making the correct diagnosis and treatment. The discovery of hepcidin in 2001 has revolutionized our understanding of iron disorders, establishing this low-molecular-weight peptide hormone as the master regulator of iron availability to the bone marrow.
Despite advances in laboratory medicine, traditional iron panel interpretation often fails to distinguish between absolute and functional iron deficiency. Hepcidin blocks critical iron flows into plasma: duodenal absorption, release from macrophages, recycling old red blood cells, and mobilization of stored iron from hepatocytes. This complex regulation explains why conventional tests may yield misleading results in patients with chronic inflammation, kidney disease, or those undergoing treatment with erythropoiesis-stimulating agents. High hepcidin levels inhibit intestinal absorption and macrophage recycling of iron, resulting in iron-restricted erythropoiesis and anemia. Furthermore, red blood cell production consumes approximately 80% of circulating iron for hemoglobin synthesis in maturing erythroblasts. This makes functional iron deficiency treatment particularly crucial when iron availability is compromised despite adequate stores.
This article examines an array of established markers of iron status alongside novel biomarkers like soluble transferrin receptor, zinc protoporphyrin, and hepcidin. Through case-based examples and evidence-based analysis, practitioners will be better able to accurately interpret iron studies, especially in complex clinical situations where traditional parameters aren’t enough.
Iron Transport and Erythropoiesis: The Basics
The human body orchestrates a remarkable iron transport system to meet the demands of red blood cell production. This complicated process involves many proteins and regulatory systems that ensure iron is used correctly and that too much free iron doesn’t cause harm.
Iron utilization in hemoglobin synthesis
Hemoglobin synthesis represents the predominant destination for iron in the human body. This essential process needs about 20 to 25 mg of iron daily to make 200 billion new red blood cells. Making red blood cells (erythropoiesis) requires the body to make about 10 billion red blood cells every hour. This makes it the body part that uses the most iron.
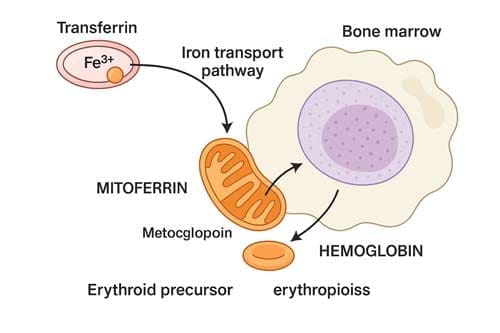
Once inside erythroid precursor cells, iron follows a carefully orchestrated pathway toward hemoglobin formation. Most transferrin-derived iron that enters the cytosol is directed to mitochondria, where heme synthesis occurs. This transport across the inner mitochondrial membrane depends primarily on mitoferrin 1 (SLC25A37) in erythroid cells. Inside mitochondria, iron becomes incorporated into protoporphyrin IX by ferrochelatase—the terminal enzyme in heme biosynthesis—ultimately forming heme.
Each mature red blood cell contains approximately 280 million molecules of hemoglobin, with each hemoglobin molecule containing four iron atoms in heme groups. Therefore, the total iron flux required to maintain erythropoiesis reaches about 2–3 x 10¹⁵ atoms of iron per second in an adult human. It highlights the enormity of the iron transport system’s task.
Role of transferrin and ferroportin in iron delivery
The iron transport system relies heavily on two key proteins: transferrin in plasma and ferroportin at cellular membranes. More than 95% of plasma iron circulates bound to transferrin, which delivers most of its cargo to erythrocyte precursors in bone marrow. However, the plasma iron pool contains only 3-4 mg of iron and must be turned over several times daily to meet erythropoietic demands.
Transferrin binding to transferrin receptor 1 (TFR1) initiates cellular iron uptake through receptor-mediated endocytosis. Subsequently, endosomal acidification causes iron to dissociate from transferrin, followed by reduction of Fe³⁺ to Fe²⁺ by the enzyme STEAP3. The divalent metal transporter 1 (DMT1) moves iron into the cytosol. Differentiating erythroid precursors express extraordinarily high levels of TFR1—approximately 800,000 receptors per cell—to maximize transferrin-bound iron uptake.
Ferroportin, the sole known cellular iron exporter, is equally crucial in iron transport. This protein mediates iron export from:
- Duodenal enterocytes absorb dietary iron
- Macrophages that recycle iron from senescent red blood cells
- Hepatocytes storing iron
- Erythrocyte precursors (under certain conditions)
Interestingly, although erythroid precursors are avid iron consumers, they also express ferroportin at the plasma membrane. During systemic iron depletion, ferroportin expression increases in these cells, potentially allowing non-erythropoietic tissues priority access to limited iron.
Erythropoietin and iron demand in red cell production
Erythropoietin (EPO), produced primarily by the kidneys, serves as the master regulator of erythropoiesis. This hormone binds to the erythropoietin receptor (EPOR) on erythroid precursors. This activates JAK2 kinase and other pathways that control how cells survive, grow, and use iron.
Notably, EPO affects how iron is used in the body in several ways. The binding of EPO to EPOR results in activation of signal transducer and activator of transcription 5 (STAT5), which increases transferrin receptor 1 expression on erythroid cells. Moreover, EPO stimulates erythroid precursors to produce erythroferrone (ERFE), a hormone that suppresses hepcidin production in the liver. Since hepcidin inhibits ferroportin, this suppression increases iron availability for erythropoiesis by enhancing iron release from storage and absorption sites.
Iron availability also influences EPO production through a feedback mechanism. When there isn’t enough iron, iron regulatory protein 1 (IRP1) binds to hypoxia-inducible factor 2α (HIF-2α), stopping its translation and lowering the production of EPO. This mechanism helps prevent hypochromic, microcytic red cell production by limiting erythropoiesis when there isn’t enough iron.
Transferrin receptor 2 (TFR2) is also an iron sensor in erythroid precursors. This protein colocalizes with EPOR to modulate EPO sensitivity based on transferrin-bound iron levels. This intricate system ensures that erythropoiesis responds appropriately to EPO stimulation and iron availability.
In situations of increased erythropoietic demand—such as blood loss, hemolysis, or exogenous EPO administration—iron requirements may increase up to 10-fold. Failure to match iron supply with this increased demand would rapidly deplete the small transferrin-bound iron pool and compromise hemoglobin synthesis and all iron-dependent processes throughout the body.
Hepcidin and the Regulation of Iron Availability
Hepcidin is the primary controller of systemic iron homeostasis. It does this through many pathways that control how much iron is available to tissues. This peptide hormone, which is made mainly by hepatocytes and has 25 amino acids, binds to the iron exporter ferroportin, causing it to be taken in and broken down. This stops iron from being absorbed by the intestines and released from storage sites. Understanding how these rules work is crucial for interpreting iron studies, especially in complicated clinical situations.
The BMP-SMAD pathway in the transcription of hepcidin
The bone morphogenetic protein-SMAD (BMP-SMAD) signalling pathway is essential to controlling hepcidin. This pathway starts with BMP ligands binding to cell surface receptors, which sets off a chain of events inside the cell. When BMP type II receptors are turned on, they add a phosphate group to type I receptors. This, in turn, adds a phosphate group to receptor-regulated SMADs (R-SMADs), which are SMAD1, SMAD5, and SMAD8. Once phosphorylated, these R-SMADs associate with common mediator SMAD4 and translocate to the nucleus, where they function as transcription factors to activate hepcidin gene expression.
Liver-specific disruption of SMAD4 in mice results in severe iron overload due to dramatically decreased hepcidin expression. Similarly, double knockout of SMAD1 and SMAD5 in hepatocytes produces hepcidin deficiency and iron overload, with triple knockout of SMAD1, SMAD5, and SMAD8 causing even more severe manifestations.
The pathway requires several key components:
- BMP ligands (primarily BMP2 and BMP6) produced by liver sinusoidal endothelial cells
- Type I receptors (ALK2 and ALK3) and type II receptors (ACVR2A and BMPR2)
- Hemojuvelin (HJV), an iron-specific co-receptor that enhances BMP signaling
- Transferrin receptor 2 (TFR2) and HFE, which increase the sensitivity of BMP receptors to their ligands
Remarkably, ALK2 and ALK3 have distinct roles—ALK3 maintains basal hepcidin expression and responds primarily to BMP2, whereas ALK2 mediates iron-dependent hepcidin upregulation and responds to BMP6.
Erythroferrone-mediated hepcidin suppression
Erythroferrone (ERFE), a hormone secreted by bone marrow erythroblasts during active erythropoiesis, powerfully suppresses hepcidin production. The erythroid regulator ERFE is produced in response to erythropoietin via the JAK2/STAT5 signaling pathway. Its primary function is to increase iron availability during heightened erythropoietic demand.
Research demonstrates that ERFE mediates hepcidin suppression during increased erythropoietic activity stimulated by endogenous or exogenous EPO and in response to blood loss. In mouse models, ablation of the ERFE gene prevented hepcidin suppression after acute blood loss and EPO injection, delaying recovery from anemia.
ERFE’s mechanism of action has been elucidated recently—it binds and sequesters certain BMP family members, most prominently BMP2, BMP6, and BMP2/6 heterodimers. Through this sequestration, ERFE inhibits BMP-SMAD signaling and reduces SMAD1/5 phosphorylation, ultimately decreasing hepcidin transcription. Furthermore, ERFE competitively inhibits BMP2/6 from binding to the type I receptor ALK3.
In β-thalassemia, a condition characterized by ineffective erythropoiesis, ERFE expression is highly increased in the bone marrow and spleen. Genetic deletion of ERFE in thalassemic mice restored normal hepcidin levels and improved iron overload, highlighting its pathological role in conditions with abnormal erythropoiesis.
Inflammation-induced hepcidin elevation via IL-6
Infection and inflammation rapidly increase hepcidin synthesis through inflammatory cytokines, chiefly interleukin-6 (IL-6). This response evolved as a host defense mechanism to limit iron availability to invading pathogens, but can lead to functional iron deficiency when chronic.
IL-6 stimulates hepcidin production through the JAK2/STAT3 signaling pathway. Once activated, STAT3 binds directly to a STAT3-binding element in the hepcidin promoter, increasing transcription. In human volunteers infused with IL-6, urinary hepcidin excretion increased over 7-fold within hours, accompanied by a 34% decrease in serum iron and a 33% decrease in transferrin saturation.
The essential role of IL-6 in inflammation-induced hepcidin production has been confirmed in knockout models. Turpentine-induced inflammation did not raise hepcidin or lower serum iron levels in IL-6-deficient mice, but it did in wild-type mice. In addition, the level of hepatic hepcidin expression in turpentine-treated IL-6 knockout mice dropped below normal.
Other inflammatory cytokines besides IL-6 can also control hepcidin, especially long-term inflammation. An intact BMP-SMAD pathway is necessary for maximum hepcidin response to inflammation, as the IL-6-STAT3 and BMP-SMAD pathways cooperate to activate hepcidin expression fully.

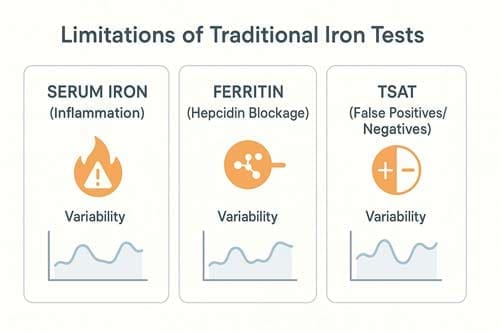
Why Traditional Iron Tests Miss Functional Deficiency
Traditional iron tests often fail to detect functional iron deficiency—a condition where iron stores are adequate but unavailable for erythropoiesis. This gap in diagnosis makes it very hard for doctors to understand iron studies in patients with many different health problems.
Ferritin as an acute-phase reactant
Interpreting serum ferritin levels requires a nuanced understanding of its dual role as an iron storage marker and an acute-phase reactant. In general, ferritin levels go up a lot during inflammatory states. For example, levels in people with C-reactive protein (CRP) >80 mg/L are more than five times higher than in people with CRP <10 mg/L. This rise happens because proinflammatory cytokines, especially interleukin-6 (IL-6), cause the liver to make acute-phase proteins like Ferritin.
Interestingly, ferritin production depends on two factors: inflammation increases ferritin mRNA transcription, but iron remains an absolute requirement for translation into protein. In states of severe iron deficiency, minimal Ferritin is produced even during inflammation. Nevertheless, in most clinical scenarios with concurrent inflammation and iron deficiency, enough iron exists to permit increased ferritin production, yielding abnormal or elevated values.
This phenomenon explains why patients with chronic kidney disease, rheumatoid arthritis, malignancy, and infections frequently exhibit elevated Ferritin despite restricted iron availability for erythropoiesis. Critically, approximately one-third of hemodialysis patients with high ferritin levels have underlying inflammation rather than iron overload. For this reason, a ferritin cutoff of 100 μg/L—rather than the traditional 15-30 μg/L threshold—is often used when inflammation is present.
Transferrin saturation variability in inflammation
Transferrin saturation (TSAT) is hard to understand because it varies significantly between people. The daily change in TSAT levels within a single person ranges from 25% to 30%, much more than the analytical change. This natural change makes it harder to monitor and understand things over time.
Additionally, inflammation profoundly affects TSAT through multiple mechanisms. Elevated hepcidin blocks iron export from enterocytes and macrophages, reducing serum iron while transferrin levels remain stable or decrease slightly. The result is decreased TSAT, which may not reflect true iron deficiency.
Low TSAT (<20%) occurs in both absolute iron deficiency and functional iron deficiency, making differential diagnosis challenging. However, despite this problem, new research suggests that TSAT may be better than Ferritin at predicting clinical outcomes. In people with heart failure, TSAT was linked to a higher risk of death, but Ferritin was not. Notably, TSAT below 20% essentially excluded bone marrow iron deficiency in heart failure patients, regardless of ferritin levels, whereas the specificity for bone marrow iron deficiency improved to 98% if associated with Ferritin <100 μg/L in non-dialysis chronic kidney disease patients.
Limitations of serum iron and TIBC in chronic disease
Serum iron measurements represent the least reliable component of the traditional iron panel. Besides being the most susceptible to laboratory contamination, serum iron exhibits diurnal variation and can normalize hours after iron ingestion. Both inflammation and absolute iron deficiency lower serum iron, rendering this test non-specific.
Total iron binding capacity (TIBC) functions as a negative acute phase reactant, decreasing during inflammation regardless of iron status. It creates particular challenges for diagnosing iron deficiency in patients with chronic inflammatory conditions. While elevated TIBC is highly specific for absolute iron deficiency (the only condition where above-normal TIBC occurs), standard or low values cannot rule out iron deficiency in inflammatory states.
Common patterns that lead to misdiagnosis include:
- Normal/high Ferritin with low TSAT in chronic inflammation (misses functional iron deficiency)
- Low-normal TIBC in inflammatory states (misses iron deficiency)
- Low serum iron in both inflammation and iron deficiency (non-specific finding)
- Normal Ferritin despite bone marrow iron depletion in chronic kidney disease
These limitations underscore why standard iron panels often fail to distinguish between absolute iron deficiency (depleted stores) and functional iron deficiency (adequate stores but impaired utilization)—a distinction with important therapeutic implications.
Functional Iron Deficiency vs Absolute Iron Deficiency
Knowing the difference between absolute and functional iron deficiency is essential to interpret iron studies correctly. How these conditions affect the body, how they are diagnosed, and how they are treated are all very different.
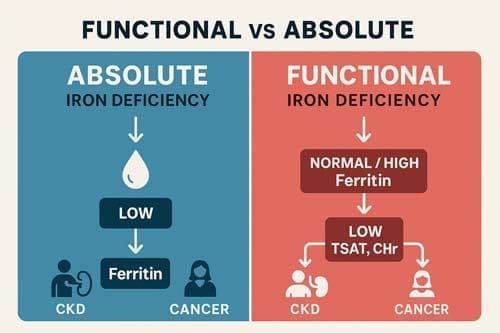
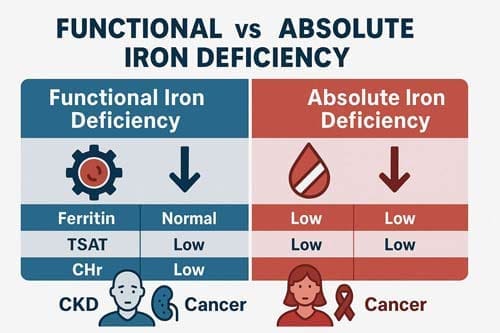
Definition and pathophysiology of functional iron deficiency
Absolute iron deficiency is when iron stores are severely low or nonexistent. When iron stores seem to be enough, but there isn’t enough iron available for erythropoiesis, this is called functional iron deficiency. Functional iron deficiency happens when there is not enough iron available to meet the body’s needs, even though there are enough iron stores. This leads to iron-restricted erythropoiesis.
Inflammation is at the heart of the pathophysiology of functional iron deficiency. IL-6, IL-1β, and lipopolysaccharide are inflammatory cytokines that make hepcidin, which breaks down ferroportin and keeps iron in macrophages in the reticuloendothelial system. This process ultimately decreases the bioavailability of plasma iron. Concurrently, inflammatory cytokines inhibit renal erythropoietin production and activity, inhibit erythropoiesis through radical formation, and promote hepatic and splenic erythrophagocytosis.
Diagnostic markers: CHr, Ret-He, %HRC
Given the limitations of traditional tests, reticulocyte-based parameters have emerged as valuable markers for detecting functional iron deficiency. Reticulocyte hemoglobin content (CHr) and reticulocyte hemoglobin equivalent (Ret-He) directly assess functional iron availability to erythropoietic tissue.
There are several significant benefits to these markers. One of the best things about them is that they can react quickly to changes in iron levels. For example, CHr levels can show improvements as little as two days after intravenous iron treatment. They are not affected by things like inflammation, infection, cancer, or liver disease like standard markers are. Because of this, you can trust them even more in tricky medical scenarios. They are also sensitive enough to find early or “subclinical” iron shortage, often before changes show up on regular iron tests.
Studies indicate that a Ret-He value below 25.7 pg predicts iron deficiency with 71.4% sensitivity and 100% specificity. For identifying functional iron deficiency specifically, a CHr value below 29 pg shows high diagnostic accuracy in patients receiving ESA therapy.
Another valuable marker, percentage of hypochromic red cells (%HRC), represents the best-established variable for identifying functional iron deficiency. Yet, practical limitations exist regarding sample stability and equipment availability.
Clinical examples: CKD, ESA therapy, chronic inflammation
Functional iron deficiency manifests prominently in several clinical scenarios. Chronic kidney disease (CKD) results from chronic inflammation, poor hepcidin clearance, and ESA therapy. Indeed, erythropoiesis-stimulating agents commonly cause functional iron deficiency because they transiently increase erythropoiesis beyond the rate at which iron can be mobilized from stores.
For patients with CKD, functional iron deficiency is typically defined as transferrin saturation <20% with Ferritin>100 μg/L in non-dialysis CKD and >200 μg/L in dialysis patients. Approximately 20-25% of dialysis patients exhibit this condition.
Other conditions characterized by functional iron deficiency include heart failure, cancer, autoimmune disease, chronic pulmonary disease, inflammatory bowel disease, and obesity. Interestingly, an estimated 15% of US adults have functional iron deficiency, exceeding the prevalence of absolute iron deficiency in all US adults except women younger than 50 years.
The management approaches differ substantially—absolute iron deficiency primarily requires iron repletion, yet functional iron deficiency often necessitates treating the underlying inflammatory condition, possibly combined with intravenous iron and/or ESA therapy.
Case-Based Interpretation of Iron Panels
Looking at real clinical situations shows how hard it can be to interpret iron studies in real life. These examples show how important it is to look at lab results in the proper context to give the best care to patients.
Case 1: CKD patient with high Ferritin and low CHr
A 56-year-old woman with stage 3 CKD (creatinine clearance 32 mL/min) comes in with tiredness, shortness of breath, and mental fog. The lab tests show that the haemoglobin level is 7.9 g/dL, the ferritin level is 89 μg/L, and the CRP level is 1.8 mg/L. This case demonstrates the common paradox in kidney disease: normal ferritin levels, even though the body doesn’t have enough iron to work correctly. For people with CKD, a ferritin level of 100 μg/L and a TSAT level of less than 20% is a better way to determine if they are iron deficient than the usual levels. Reticulocyte haemoglobin content (CHr) is a better diagnostic tool because levels below 29 pg show functional iron deficiency even when ferritin levels look normal. So, even though the iron stores seem normal, intravenous iron supplementation is still a good idea.
Case 2: Cancer patient with anemia and regular iron stores
A patient with colorectal cancer presents with hemoglobin 10.5 g/dL, ferritin 422 ng/mL, and low transferrin saturation. Cancer-associated inflammation drives functional iron deficiency through cytokine-mediated hepcidin upregulation. Among cancer patients, the prevalence of iron deficiency reaches 42.6%, with particularly high rates in pancreatic (63.2%), colorectal (52.2%), and lung cancers (51.3%). Remarkably, 81.9% of iron-deficient cancer patients present with functional rather than absolute deficiency. Standard ferritin cutoffs fail in this population; physicians should consider functional iron deficiency when TSAT is <20% with ferritin levels up to 800 ng/mL.
Case 3: IRIDA with elevated hepcidin and low transferrin saturation
A pair of fraternal twins presents with severe microcytic anemia, hypoferremia, and resistance to oral iron therapy. Genetic testing reveals compound heterozygous TMPRSS6 mutations causing iron-refractory iron deficiency anemia (IRIDA). IRIDA is associated with inappropriately high hepcidin levels relative to iron status due to matriptase-2 dysfunction. The TSAT/hepcidin ratio provides extraordinary diagnostic utility—patients with IRIDA show ratios below 5.6%/nM with 100% sensitivity and specificity. Primarily, parenteral iron therapy offers the best clinical response, as oral supplementation fails due to hepcidin-mediated blockade of intestinal iron absorption.
Emerging Tools for Functional Iron Deficiency Detection
Beyond traditional iron panels, newer laboratory tests offer superior detection of functional iron deficiency—a critical advance for patients with misleading conventional results. These emerging tools provide earlier identification and more accurate differentiation of iron-restricted states.
Hepcidin assays: mass spectrometry vs immunoassay
Different ways of measuring hepcidin have various levels of selectivity for biologically active forms. Liquid chromatography tandem mass spectrometry (LC-MS/MS) only measures bioactive hepcidin-25, but immunoassays find all hepcidin isoforms. This difference is essential in the clinic because minor, non-biologically active isoforms increase significantly during inflammatory conditions.
Comparisons between these methods reveal consistent patterns—immunoassay measurements typically exceed LC-MS/MS values, with differences magnified during inflammation. In acute pneumonia, immunoassay measured 37% higher hepcidin concentrations than LC-MS/MS for samples below 200 ng/mL, expanding to 78% higher for concentrations above 200 ng/mL. Though LC-MS/MS remains the gold standard, point-of-care immunoassays offer faster results using general clinical laboratory equipment.
CHr and Ret-He as early markers of iron-restricted erythropoiesis
Reticulocyte hemoglobin content (CHr or Ret-He) provides an indirect measure of functional iron available for new red blood cell production over the previous 3-4 days. Unlike traditional markers, CHr reflects real-time erythropoiesis, responding rapidly to therapy, increasing within 2-4 days after intravenous iron administration.
Importantly, CHr remains unaffected by inflammation, infection, malignancy, or liver disease. A CHr <28 pg diagnostic threshold effectively identifies functional iron deficiency in anemic patients. This parameter works exceptionally well for monitoring patients receiving erythropoietin therapy, promptly detecting iron-restricted erythropoiesis.
Zinc protoporphyrin and soluble transferrin receptor (sTfR)
Zinc protoporphyrin (ZPP) forms primarily when the iron supply to erythropoietic tissue is insufficient. It provides long-term information (approximately 3 months) on iron status. Similarly, soluble transferrin receptor (sTfR) increases during iron-deficient erythropoiesis.
Comparisons between these markers show that ZPP demonstrates greater sensitivity in early iron deficiency. Even with excellent correlation between ZPP and sTfR (r = 0.86; P < 0.0001), elevated sTfR appears only in 25% of cases with mild iron-deficient erythropoiesis. Conversely, sTfR maintains analytical reliability during inflammation (AUCROC = 0.92).
The sTfR/log10 ferritin index provides outstanding diagnostic performance (area under the curve 1.00), making these parameters valuable complements rather than competitors in comprehensive iron assessment.
Conclusion
Iron panels have been the standard way to find and keep track of iron levels for a long time, but their limitations become clear when faced with complicated medical situations. This article examined how hepcidin-mediated iron regulation changes our thoughts about iron-restricted states, especially functional iron deficiency. Undoubtedly, the distinction between absolute and functional iron deficiency represents more than academic interest—it directly impacts clinical management and patient outcomes.
Modern iron study interpretation, therefore, demands recognition of fundamental physiological principles. First, hepcidin is the master regulator, dictating iron flow regardless of storage adequacy. Second, inflammation profoundly alters traditional markers through multiple mechanisms. Finally, erythropoietic demand creates a dynamic environment where iron sufficiency exists on a spectrum rather than as a binary state.
Consequently, alternative diagnostic approaches merit consideration in challenging cases. Reticulocyte haemoglobin content (CHr) and its equivalents (Ret-He) have unique benefits because they quickly respond to changes in iron levels and are unaffected by inflammation. The percentage of hypochromic red cells (%HRC) also gives us helpful information about how much functional iron is available. New tools like hepcidin assays, zinc protoporphyrin, and soluble transferrin receptors make it easier to get accurate diagnoses.
In the end, the clinical context is still the most important thing. Chronic kidney disease, cancer, and inflammatory conditions make iron metabolism more complicated in different ways, so each needs its way of interpreting the data. Depending on the situation, the right ferritin level can range from 30 μg/L in healthy people to 300 μg/L in people on haemodialysis. It is also essential to look at transferrin saturation in context rather than using strict cutoff values.
Because iron metabolism diagnostics are changing, doctors must go beyond simple algorithms. Laboratory tests give us essential information, but a complete interpretation must consider physiological principles, how the patient looks and feels, and the right diagnostic thresholds for different groups of patients. Also, using the same method for sequential monitoring gives more accurate diagnoses than taking measurements one at a time.
Healthcare providers who understand iron regulation in this way can better spot and treat both absolute and functional iron deficiency. This advanced method makes it easier to manage anemia in many patients, from those with chronic inflammatory diseases to those taking erythropoiesis-stimulating drugs. After all, it is still essential to correctly interpret iron studies to make accurate diagnoses and provide the best care for patients.

Frequently Asked Questions:
FAQs
Q1. What is functional iron deficiency, and how does it differ from absolute iron deficiency? Functional iron deficiency occurs when there are adequate iron stores in the body, but the iron is not available for erythropoiesis (red blood cell production). This differs from absolute iron deficiency, where iron stores are depleted. Functional iron deficiency is often caused by inflammation or chronic diseases that affect iron metabolism.
Q2. Why do traditional iron tests sometimes fail to detect functional iron deficiency? Traditional iron tests like Ferritin can give false results because it is an acute-phase reactant that rises during inflammation, regardless of the person’s iron levels. Inflammation can also change transferrin saturation, making it hard to tell how much iron is available to make red blood cells.
Q3. What are some emerging tools for detecting functional iron deficiency? Newer diagnostic tools include reticulocyte hemoglobin content (CHr or Ret-He), which provides a real-time measure of iron available for erythropoiesis, and hepcidin assays, which can help assess iron regulation. Other markers, like zinc protoporphyrin and soluble transferrin receptor, can also provide information about iron status.
Q4. How does chronic kidney disease (CKD) affect iron metabolism and interpretation of iron studies? CKD patients often have functional iron deficiency due to chronic inflammation, poor hepcidin clearance, and the effects of erythropoiesis-stimulating agent therapy. In these patients, higher ferritin thresholds (e.g., >100 μg/L for non-dialysis and >200 μg/L for dialysis patients) are used to diagnose iron deficiency when combined with low transferrin saturation.
Q5. What role does hepcidin play in iron regulation, and how does it impact iron availability? Hepcidin is the master regulator of iron metabolism. It stops iron from being absorbed in the intestine and released from storage sites by breaking down ferroportin, the only known iron exporter in cells. High levels of hepcidin, which are often caused by inflammation, can cause functional iron deficiency by making it harder for the body to use iron for erythropoiesis, even though there are enough iron stores.
References:
[1] – https://www.ohsu.edu/sites/default/files/2019-08/Iron Deficiency.pdf
[2] – https://pmc.ncbi.nlm.nih.gov/articles/PMC6708300/
[3] – https://www.ncbi.nlm.nih.gov/books/NBK519570/
[4] – https://pmc.ncbi.nlm.nih.gov/articles/PMC2893236/
[6] – https://onlinelibrary.wiley.com/doi/10.1111/trf.15577
[7] – https://pmc.ncbi.nlm.nih.gov/articles/PMC11423176/
[8] – https://pmc.ncbi.nlm.nih.gov/articles/PMC10552342/
[10] – https://pmc.ncbi.nlm.nih.gov/articles/PMC8836508/
[12] – https://www.ncbi.nlm.nih.gov/books/NBK559119/
[13] – https://pmc.ncbi.nlm.nih.gov/articles/PMC12129200/
[14] – https://www.ahajournals.org/doi/10.1161/CIRCHEARTFAILURE.124.011728
[15] – https://pmc.ncbi.nlm.nih.gov/articles/PMC2754511/
[16] – https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2823909
[17] – https://pmc.ncbi.nlm.nih.gov/articles/PMC11825113/
[18] – https://onlinelibrary.wiley.com/doi/10.1111/bjh.12311
[19] – https://pmc.ncbi.nlm.nih.gov/articles/PMC10319843/
[20] – https://www.ajkd.org/article/S0272-6386(21)00955-0/fulltext
[21] – https://pmc.ncbi.nlm.nih.gov/articles/PMC7922992/
[22] – https://pmc.ncbi.nlm.nih.gov/articles/PMC6247786/
[23] – https://pmc.ncbi.nlm.nih.gov/articles/PMC6315653/
[24] – https://www.sciencedirect.com/science/article/pii/S0923753419366608
[25] – https://www.bloodresearch.or.kr/journal/view.html?uid=2677&vmd=Full
[26] – https://onlinelibrary.wiley.com/doi/full/10.1002/ccr3.6401
[27] – https://www.sciencedirect.com/science/article/pii/S2405844023011039
[28] – https://pubmed.ncbi.nlm.nih.gov/18027835/
[29] – https://academic.oup.com/clinchem/article-abstract/50/7/1240/5640129
[30] – https://pubmed.ncbi.nlm.nih.gov/16146537/
[31] – https://pmc.ncbi.nlm.nih.gov/articles/PMC1633791/

