DOSAGE AND ADMINISTRATION [Oral Capsules]
------------------------------------------------------------------------------------------------------
The optimal daily dose of calcitriol must be carefully determined for each patient. Calcitriol Capsules should be administered orally. Calcitriol therapy should always be started at the lowest possible dose and should not be increased without careful monitoring of serum calcium.
The effectiveness of calcitriol therapy is predicated on the assumption that each patient is receiving an adequate but not excessive daily intake of calcium. Patients are advised to have a dietary intake of calcium at a minimum of 600 mg daily. The U.S. RDA for calcium in adults is 800 mg to 1200 mg. To ensure that each patient receives an adequate daily intake of calcium, the physician should either prescribe a calcium supplement or instruct the patient in proper dietary measures.
Because of improved calcium absorption from the gastrointestinal tract, some patients on calcitriol may be maintained on a lower calcium intake. Patients who tend to develop hypercalcemia may require only low doses of calcium or no supplements at all.
During the titration period of treatment with calcitriol, serum calcium levels should be checked at least twice weekly. When the optimal dosage of calcitriol has been determined, serum calcium levels should be checked every month (or as given below for individual indications). Samples for serum calcium estimation should be taken without a tourniquet.
Dialysis Patients
The recommended initial dose of calcitriol is 0.25 mcg/day. If a satisfactory response in the biochemical parameters and clinical manifestations of the disease state is not observed, dosage may be increased by 0.25 mcg/day at 4 to 8 week intervals. During this titration period, serum calcium levels should be obtained at least twice weekly, and if hypercalcemia is noted, the drug should be immediately discontinued until normocalcemia ensues. Phosphorus, magnesium, and alkaline phosphatase should be determined periodically.
Patients with normal or only slightly reduced serum calcium levels may respond to calcitriol doses of 0.25 mcg every other day. Most patients undergoing hemodialysis respond to doses between 0.5 and 1 mcg/day.
Oral calcitriol may normalize plasma ionized calcium in some uremic patients, yet fail to suppress parathyroid hyperfunction. In these individuals with autonomous parathyroid hyperfunction, oral calcitriol may be useful to maintain normocalcemia, but has not been shown to be adequate treatment for hyperparathyroidism.
Hypoparathyroidism
The recommended initial dosage of calcitriol is 0.25 mcg/day given in the morning. If a satisfactory response in the biochemical parameters and clinical manifestations of the disease is not observed, the dose may be increased at 2- to 4-week intervals. During the dosage titration period, serum calcium levels should be obtained at least twice weekly and, if hypercalcemia is noted, calcitriol should be immediately discontinued until normocalcemia ensues. Careful consideration should also be given to lowering the dietary calcium intake. Serum calcium, phosphorus, and 24-hour urinary calcium should be determined periodically.
Most adult patients and pediatric patients age 6 years and older have responded to dosages in the range of 0.5 mcg to 2 mcg daily. Pediatric patients in the 1 to 5 year age group with hypoparathyroidism have usually been given 0.25 mcg to 0.75 mcg daily. The number of treated patients with pseudohypoparathyroidism less than 6 years of age is too small to make dosage recommendations.
Malabsorption is occasionally noted in patients with hypoparathyroidism; hence, larger doses of calcitriol may be needed.
Predialysis Patients
The recommended initial dosage of calcitriol is 0.25 mcg/day in adults and pediatric patients 3 years of age and older. This dosage may be increased if necessary to 0.5 mcg/day.
For pediatric patients less than 3 years of age, the recommended initial dosage of calcitriol is 10 to 15 ng/kg/day.
DOSAGE AND ADMINISTRATION [INFECTION]
------------------------------------------------------------------------------------------------------
The optimal dose calcitriol injection must be carefully determined for each patient.
The effectiveness of calcitriol injection therapy is predicated on the assumption that each patient is receiving an adequate and appropriate daily intake of calcium. The RDA for calcium in adults is 800 mg. To ensure that each patient receives an adequate daily intake of calcium, the physician should either prescribe a calcium supplement or instruct the patient in proper dietary measures.
The recommended initial dose of calcitriol injection, depending on the severity of the hypocalcemia and/or secondary hyperparathyroidism, is 1 mcg (0.02 mcg/kg) to 2 mcg administered three times weekly, approximately every other day. Doses as small as 0.5 mcg and as large as 4 mcg three times weekly have been used as an initial dose. If a satisfactory response is not observed, the dose may be increased by 0.5 to 1 mcg at two to four week intervals. During this titration period, serum calcium and phosphorus levels should be obtained at least twice weekly. If hypercalcemia or a serum calcium times phosphate product greater than 70 is noted, the drug should be immediately discontinued until these parameters are appropriate. Then, the calcitriol injection dose should be reinitiated at a lower dose. Doses may need to be reduced as the PTH levels decrease in response to the therapy. Thus, incremental dosing must be individualized and commensurate with PTH, serum calcium and phosphorus levels.
The following is a suggested approach in dose titration:
| PTH Levels |
Calcitriol Dose |
| the same or increasing |
increase |
| decreasing by less than 30% |
increase |
| decreasing by more than 30% but less than 60% |
maintain |
| decreasing by more than 60% |
decrease |
| one and one-half to three times the upper limit of normal |
maintain |
Supplied:
Capsule (Rocaltrol®): 0.25 mcg, 0.5 mcg.
Injection, solution: 1 mcg/mL (1 mL); 2 mcg/mL (2 mL)
Calcijex®: 1 mcg/mL (1 mL).
Solution, oral (Rocaltrol®): 1 mcg/mL (15 mL).
ADMINISTRATION — May be administered without regard to food. Give with meals to reduce GI problems. May be administered as a bolus dose I.V. through the catheter at the end of hemodialysis.
COMPATIBILITY — Stable in D5W, NS, sterile water for injection.
CLINICAL PHARMACOLOGY
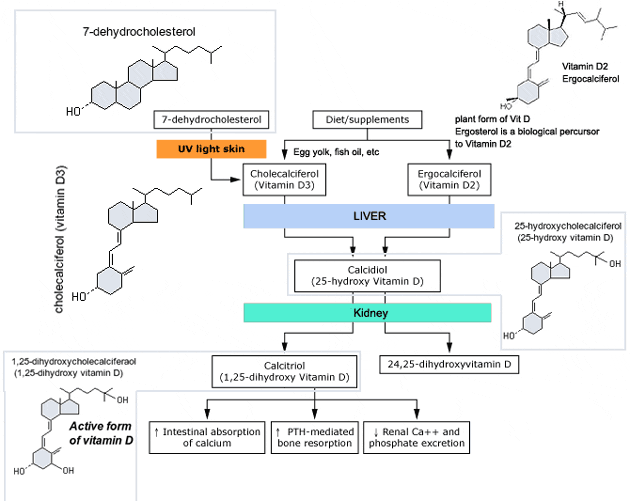
Man's natural supply of vitamin D depends mainly on exposure to the ultraviolet rays of the sun for conversion of 7-dehydrocholesterol in the skin to vitamin D3 (cholecalciferol). Vitamin D3 must be metabolically activated in the liver and the kidney before it is fully active as a regulator of calcium and phosphorus metabolism at target tissues. The initial transformation of vitamin D3 is catalyzed by a vitamin D3-25-hydroxylase enzyme (25-OHase) present in the liver, and the product of this reaction is 25-hydroxyvitamin D3 [25-(OH)D3]. Hydroxylation of 25-(OH)D3 occurs in the mitochondria of kidney tissue, activated by the renal 25-hydroxyvitamin D3-1 alpha-hydroxylase (alpha-OHase), to produce 1,25-(OH)2D3 (calcitriol), the active form of vitamin D3. Endogenous synthesis and catabolism of calcitriol, as well as physiological control mechanisms affecting these processes, play a critical role regulating the serum level of calcitriol. Physiological daily production is normally 0.5 to 1 mcg and is somewhat higher during periods of increased bone synthesis (eg, growth or pregnancy).
INDICATIONS AND USAGE
Predialysis Patients
Calcitriol Capsules are indicated in the management of secondary hyperparathyroidism and resultant metabolic bone disease in patients with moderate to severe chronic renal failure (Ccr 15 to 55 mL/min) not yet on dialysis. In children, the creatinine clearance value must be corrected for a surface area of 1.73 square meters. A serum iPTH level of ≥100 pg/mL is strongly suggestive of secondary hyperparathyroidism.
Dialysis Patients
Calcitriol Capsules are indicated in the management of hypocalcemia and the resultant metabolic bone disease in patients undergoing chronic renal dialysis. In these patients, calcitriol administration enhances calcium absorption, reduces serum alkaline phosphatase levels, and may reduce elevated parathyroid hormone levels and the histological manifestations of osteitis fibrosa cystica and defective mineralization.
Hypoparathyroidism Patients
Calcitriol Capsules are also indicated in the management of hypocalcemia and its clinical manifestations in patients with postsurgical hypoparathyroidism, idiopathic hypoparathyroidism, and pseudohypoparathyroidism.
CONTRAINDICATIONS
Calcitriol Capsules should not be given to patients with hypercalcemia or evidence of vitamin D toxicity. Use of Calcitriol Capsules in patients with known hypersensitivity to Calcitriol Capsules (or drugs of the same class) or any of the inactive ingredients is contraindicated.
PHARMACODYNAMICS / KINETICS
Onset of action: ~2-6 hours.
Duration: 3-5 days.
Absorption: Oral: Rapid.
Protein binding: 99.9%.
Metabolism: Primarily to 1,24,25-trihydroxycholecalciferol and 1,24,25-trihydroxy ergocalciferol.
Half-life elimination: 3-8 hours.
Dosing (Adults):
Individualize dosage to maintain calcium levels of 9-10 mg/dL.
Renal failure:
Oral: 0.25 mcg/day or every other day (may require 0.5-1 mcg/day).
I.V.: 0.5 mcg (0.01 mcg/kg) 3 times/week; most doses in the range of 0.5-3 mcg (0.01-0.05 mcg/kg) 3 times/week.
Hypoparathyroidism/pseudohypoparathyroidism:
Oral: 0.5 to 2 mcg/day.
Vitamin D-dependent rickets: Oral: 1 mcg once daily.
Vitamin D-resistant rickets (familial hypophosphatemia): Oral: Initial: 0.015 to 0.02 mcg/kg once daily; maintenance: 0.03-0.06 mcg/kg once daily; maximum dose: 2 mcg once daily. |