The Hidden Cost of Skipping Frailty Assessment: New Research Reveals
Please like and subscribe if you enjoyed this video 🙂
Introduction
Frailty assessment remains critically underutilized in healthcare settings despite affecting approximately 15% of noninstitutionalized adults in the United States . Global estimates of frailty range dramatically from 3.5% to 27.3%, with prevalence generally increasing with age . Recent evidence demonstrates that females experience higher rates of frailty (15–29%) compared to males (11–20%) . These statistics underscore the widespread nature of a condition that often goes unrecognized.
Despite its prevalence, frailty syndrome receives inadequate attention in clinical practice. A systematic review estimated that 3-5% of deaths could have been delayed if healthcare providers had recognized and prevented frailty syndrome . Furthermore, while numerous assessment methods exist—with the Frailty Index (41.0%), Clinical Frailty Scale (23.3%), and Fried Frailty Phenotype (9.3%) being the most common—their implementation often falls short of their potential . Though healthcare practitioners conduct frailty assessments to evaluate associations with adverse outcomes or validate specific tools, only 0.4% of studies measured frailty to prospectively adapt healthcare provision . This striking disconnect between assessment and action represents a significant missed opportunity in patient care.
The rising prevalence of frailty—attributed both to increasing longevity and enhanced detection capabilities—demands greater awareness among healthcare personnel . This article examines the multifaceted consequences of overlooking frailty assessment in clinical settings, from increased hospital readmissions to economic burdens and missed opportunities for early intervention. Additionally, it explores the limitations of current assessment tools and offers practical recommendations for integration into routine clinical practice.

Why Frailty Assessment Matters in Modern Healthcare
Modern healthcare increasingly recognizes frailty as a cornerstone of geriatric medicine and a crucial determinant of patient outcomes. Defined as a multifactorial medical syndrome characterized by reduced endurance and decreased physiological ability, frailty creates vulnerability to adverse health events and mortality [1]. Early identification through proper assessment tools has become essential for effective patient management and resource allocation.
Frailty as a predictor of adverse outcomes
Frailty functions as a powerful predictor of multiple adverse health outcomes across healthcare settings. Research consistently demonstrates that frail older adults face substantially elevated risks compared to their non-frail counterparts, including:
-
1.3 to 2.6-fold increased risk of worsening mobility and decreased activities of daily living [2]
-
Higher rates of falls, disability, and hospitalization [2]
-
3.49 times higher overall mortality risk compared to non-frail individuals [3]
-
2.14 times higher mortality risk compared to pre-frail individuals [3]
-
8.20 times higher in-hospital mortality compared to non-frail patients [3]
The predictive power of frailty extends beyond immediate health risks. Studies reveal that frail individuals experience markedly longer hospital stays, averaging 13.5 days compared to 10.5 days for pre-frail and 8.3 days for non-frail patients [3]. Moreover, frailty predicts functional decline at discharge and mortality in both medium and long-term timeframes [3].
Notably, even pre-frail status carries substantial risks. One systematic review found pre-frail individuals have 1.51 times higher risk for functional decline than non-frail counterparts [3]. This underscores why assessment matters not only for identifying frailty but also pre-frailty, particularly since community-dwelling older adults represent the vast majority of this population [4].
The Clinical Frailty Scale (CFS) has emerged as a vital tool for predicting specific outcomes, including survival of COVID-19 patients and outcomes following coronary artery bypass grafting [5]. In hospital settings, the utility of frailty assessment becomes even more apparent, with moderate to severe frailty increasing length of stay, likelihood of discharge to locations other than home, and mortality risk [6].
Difference between frailty and chronological age
Perhaps the most compelling reason for implementing frailty assessment lies in understanding that frailty and chronological age, although related, represent fundamentally different concepts [7]. Biological age assessed through frailty tools often proves more predictive of adverse outcomes than chronological age alone [7].
The heterogeneity of aging becomes apparent when examining frailty indices. Studies show that frailty demonstrates an age-dependent exponential increase with mortality [5]. However, after adjusting for age and sex, the frailty index outperforms other aging markers in predicting mortality, especially among the oldest adults [5]. This suggests frailty assessment captures something chronological age cannot—the actual physiological reserves and resilience of the individual patient.
Research confirms this distinction through multiple approaches. In intensive care settings, for instance, biological age acceleration (measured through epigenetic markers) correlates with frailty and both predict mortality, with chronological age showing poor predictive performance by comparison [5]. This supports the hypothesis that frailty essentially represents a model of biological rather than chronological age [5].
The dynamic nature of frailty further distinguishes it from the unidirectional progression of chronological age. Frailty status can improve, worsen, or remain stable over time [2]. Women and individuals with better socioeconomic conditions show higher likelihood of improving their frailty status [2], whereas conditions like dementia and cancer limit improvement possibilities [2]. This plasticity offers opportunities for intervention that relying on chronological age alone would miss.
Consequently, frailty assessment provides clinicians with actionable information beyond what chronological age can offer, allowing for personalized care approaches based on physiological rather than simply temporal metrics.
The Clinical Cost of Skipping Frailty Assessment
Failing to incorporate frailty assessment tools into regular clinical practice creates substantial negative consequences for both patients and healthcare systems. Beyond theoretical concerns, tangible clinical costs manifest in several measurable ways when healthcare providers skip this essential diagnostic step.
Increased hospital readmissions
The absence of proper frailty assessment directly correlates with higher hospital readmission rates. Patients with frailty face substantially elevated readmission risks compared to their non-frail counterparts. One study revealed that frail patients had a 30-day readmission or death rate of 24.1% versus just 13.8% for non-frail individuals [3]. Those with moderate to severe frailty demonstrated an even higher event rate of 31.0%, with an adjusted odds ratio of 2.19 for readmission or death [3].
The readmission pattern varies over time. Within the first week after discharge, readmission rates show minimal difference between frail and non-frail patients (7% vs. 6%) [4]. Nevertheless, the gap widens dramatically in subsequent weeks—between 8-30 days post-discharge, frail patients experience 6% more readmissions (17% vs. 11%), and between 31-90 days, they show 7% higher rates (19% vs. 12%) [4]. Approximately 40% of frail patients revisit emergency departments within 90 days post-discharge [4].
These readmissions aren’t merely inconvenient—they represent deterioration in patient condition and create substantial financial burden. In the United States alone, unplanned readmissions cost between $15-20 billion annually [3]. Hence, skipping frailty assessment contributes to this expenditure by failing to identify those at highest risk.
Delayed diagnosis of geriatric syndromes
Omitting frailty assessment frequently leads to delayed identification of underlying geriatric syndromes. Research indicates that patients with frailty or presenting with geriatric syndromes receive less timely care. Those with mild, moderate, and severe frailty are less likely to be assessed within 4 hours of arrival (adjusted odds ratios: 0.79, 0.67, and 0.75 respectively) [8]. Similarly, patients with geriatric syndromes face a decreased likelihood of prompt assessment (adjusted OR 0.66) [8].
Furthermore, consultant review—a crucial quality indicator—occurs less frequently for these patients. Moderate and severe frailty patients experience 25% lower odds of meeting consultant review standards [8]. Patients with geriatric syndromes face even greater disadvantages, with 41% lower odds of achieving this quality benchmark [8].
This delay in recognition contributes to care inequalities throughout the acute care system. Without proper frailty screening, healthcare providers often miss crucial conditions including cognitive impairment, mood disorders, urinary incontinence, malnutrition, and gait impairments [5]. Approximately half of elderly patients lack routine cognitive testing, while only one-quarter receive annual falls screening [5]. Screening remains particularly poor for dementia, depression, and osteoporosis [5].
Missed early intervention opportunities
Perhaps the most costly aspect of skipping frailty assessment lies in forfeited intervention opportunities. Frailty, especially in its earlier stages, often responds to appropriate interventions [9]. Without assessment, clinicians miss critical chances to implement preventative measures that could reduce hospitalization risk, prevent institutionalization, and improve quality of life [5].
The comprehensive geriatric assessment and management of geriatric conditions have demonstrated effectiveness in preventing functional decline [5]. Key missed opportunities include:
-
Prehabilitation planning: Failure to identify high-risk surgical patients means losing chances for preoperative optimization [9]
-
Appropriate treatment selection: Without frailty status information, clinicians risk over-treatment or under-treatment [10]
-
Early rehabilitation referrals: Unrecognized frailty prevents timely initiation of physical therapy and occupational therapy interventions [11]
-
Care transitions optimization: Poor frailty recognition leads to inappropriate discharge planning and inadequate post-discharge support [3]
The absence of frailty assessment additionally impairs risk stratification, limiting clinicians’ ability to make informed decisions about treatment intensity and goals of care. This creates a cascade of missed preventative opportunities, often culminating in what one study termed “inequalities in the acute care system” for people with frailty [8].
Economic Burden of Unrecognized Frailty
The financial implications of unrecognized frailty extend far beyond clinical outcomes, creating substantial economic strain on healthcare systems. Research reveals a clear connection between frailty status and healthcare expenditure, with systematic assessment offering opportunities to mitigate these costs through early intervention and appropriate resource allocation.
Longer hospital stays and ICU admissions
Unidentified frailty directly contributes to extended hospitalizations and intensive care requirements. Data consistently shows frail patients experience significantly longer inpatient stays compared to their non-frail counterparts. In hospital settings, frail patients average 8 days in the ICU versus 6 days for non-frail individuals [2]. This pattern extends to overall hospital length of stay (LOS), with frail patients remaining hospitalized for a median of 20 days compared to just 12 days for non-frail patients [2].
The disparity becomes even more pronounced during prolonged ICU admissions. Among ICU “long-stayers,” frail patients demonstrated increased mortality at 6 months (34%) compared to pre-frail (21%) and non-frail (13%) individuals [2]. Furthermore, these extended stays often necessitate additional support post-discharge, with 53% of frail patients requiring nursing assistance at home, versus 41% of pre-frail and 24% of non-frail patients [2].
This extended LOS phenomenon appears consistently across different patient populations. For instance, in a study examining fall-related injuries, linear regression analysis indicated frail patients stayed an average of 1.2 days longer than non-frail patients after controlling for age and sex [12]. Another study examining post-CABG patients found frailty associated with an average increase of 1.3 days in hospital stay [13].
Higher healthcare costs due to complications
In a UK-based study, the extra annual healthcare cost per person was £561.05 for mild frailty, £1,208.60 for moderate frailty, and £2,108.20 for severe frailty—totaling an additional £5.8 billion annually across the UK healthcare system [1].
Hospital admission costs constitute approximately 67% of total healthcare expenditure for these patients, with specialist visits accounting for 29% and emergency visits representing 4% [14]. The economic impact becomes particularly evident in surgical settings, where adjusted hospitalization costs for frail patients can be nearly double those of non-frail patients [7]. The greatest absolute differences in adjusted costs occur following complex operations such as perforated ulcer repair ($24,600 difference) and small bowel resection ($21,600 difference) [7].
Impact on resource allocation and staffing
Unrecognized frailty creates substantial challenges for healthcare resource allocation and staffing models. As frailty severity increases, emergency hospital admission incidence rate ratios rise dramatically—1.64 for mild frailty, 2.45 for moderate frailty, and 3.16 for severe frailty compared to non-frail status [1]. More strikingly, the incidence rate ratio for inpatient days reaches 7.26 for severe frailty [1].
Efficient staffing models become essential for addressing these challenges. The high-MD model typically incurs the highest clinician labor costs due to higher average wages for the provider mix [6]. Conversely, practices with NP/PA or team-based staffing may be more likely to perform assessments of social needs and provide enhanced care for frail older adults [6].
Team-based practices, though relatively uncommon, demonstrate notable efficiency—providing care with similar staff FTEs but lower labor costs while offering a wider range of services specifically designed for older adults [6]. This approach may present a viable economic solution for addressing the resource intensity associated with frail populations.
Without proper frailty assessment tools integrated into clinical workflows, these economic burdens remain largely unaddressed. Early identification through standardized assessment could facilitate appropriate resource allocation, potentially reducing the substantial economic impact currently observed across healthcare systems.
Missed Risk Stratification Without a Frailty Score
Proper risk stratification represents a cornerstone of effective surgical decision-making, yet without frailty assessment, clinicians operate with a critical blind spot. Research demonstrates that omitting frailty scores leaves practitioners unable to accurately predict surgical outcomes, resulting in inappropriate procedure selection and missed intervention opportunities.
Failure to identify high-risk surgical patients
The absence of frailty assessment prior to surgical intervention results in flawed risk prediction across all procedure types. Patients with frailty show substantially elevated mortality following even minor surgical procedures, with 30-day mortality rates reaching 1.55% after lowest-stress operations (e.g., cystoscopy) and 5.13% following moderate-stress procedures (e.g., laparoscopic cholecystectomy) [3]. These rates surpass the 1% threshold traditionally defining high-risk surgery [3].
For very frail patients, the consequences prove far more serious:
-
10.34% 30-day mortality after lowest-stress procedures [3]
-
18.74% 30-day mortality after moderate-stress procedures [3]
-
43.00% 180-day mortality after moderate-stress procedures [3]
Without frailty assessment tools such as the Risk Analysis Index (RAI), clinicians cannot identify these high-risk individuals. As one study noted, “frailty should be assessed for any patient considering surgery and frailty-associated risks be discussed with patients in a robust process of shared decision-making” [3].
Inadequate prehabilitation planning
Failing to identify frailty before surgery eliminates crucial opportunities for prehabilitation—interventions designed to optimize patients’ physiological status preoperatively. Prehabilitation programs targeting specific frailty components have demonstrated positive effects on postoperative complications and functional recovery in frail older patients [4]. In fact, one meta-analysis found a 16% reduction in total complications among frail patients who received prehabilitation interventions [4].
Yet these benefits remain inaccessible without proper screening. Preoperative frailty identification enables clinicians to initiate early rehabilitation, provide caregiver support, or consider palliative alternatives in high-risk cases [15]. Furthermore, prehabilitation accelerates functional skill recovery and reduces rates of postoperative delirium and cognitive disorders [16].
Clearly, comprehensive prehabilitation planning cannot occur without initial frailty assessment using validated tools. Researchers have emphasized that “understanding frailty in the context of operative risk allows for targeted interventions such as nutritional optimization, exercise plans, or palliative care consultation in advance of elective surgery” [17].
Over-treatment or under-treatment scenarios
Perhaps most concerning, absence of frailty scoring creates dual risks of both over-treatment and under-treatment. Regarding over-treatment, frail patients often receive therapies inappropriate for their physiological reserve. As research indicates, even “low- to moderate-stress procedures such as cystoscopy were high-risk in frail patients” [15].
Simultaneously, chronological age frequently becomes the sole determinant for treatment decisions without frailty assessment, leading to under-treatment. Studies reveal that “surgeons should base care decisions on multidimensional health status and frailty rather than chronological age” [18]. Indeed, experts emphasize that withholding guideline-recommended therapy based solely on age “exemplifies a lack of justice” [8].
This bidirectional risk was highlighted in a consensus statement concluding it is “unethical to make a treatment recommendation without formal assessment of patient frailty” [8]. Therefore, implementing standardized frailty screening tools provides the only reliable method for balancing aggressive intervention against patient vulnerability while avoiding age-based discrimination.
Comparing Outcomes: With vs Without Frailty Assessment
Examining the quantifiable impact of implementing frailty assessment reveals striking contrasts in patient outcomes across multiple healthcare settings. Research consistently demonstrates that properly identifying frailty status transforms clinical decision-making and directly affects survival, recovery, and quality of life.
Mortality and morbidity differences
Patient survival rates differ dramatically when frailty assessment guides clinical care. Evidence shows frail individuals face a 75% higher risk of major cardiovascular events (adjusted HR = 1.75), a 53% higher risk of all-cause mortality (adjusted HR = 1.53), and a 58% higher risk of serious adverse events (adjusted HR = 1.58) compared to robust patients [5]. In emergency general surgery, frailty assessment identified patients with nearly 10% mortality rates versus just 3.79% in non-frail counterparts [19].
The disparity becomes even more apparent in specific populations:
-
Frail emergency department patients show 7.9% 30-day mortality versus 0.9% for non-frail patients [20]
-
Frail ICU patients experience 27% ICU mortality versus 22% for non-frail patients [21]
-
Frail surgical patients demonstrate 29.2% complication rates compared to 14.4% in non-frail patients [22]
Notably, systematic frailty screening programs yield tangible improvements. One healthcare system implementing a Frailty Screening Initiative using the Risk Analysis Index saw 1-year postoperative mortality decrease from 3.9% to 3.3% systemwide, with an even more dramatic reduction in frail patients specifically (from 20.2% to 16.0%) [11].
Functional decline and quality of life metrics
Beyond survival, frailty assessment identifies patients at risk for functional deterioration. After vascular surgery, frail patients experience a substantial decline in abilities that persists for extended periods. One study documented that frail individuals had 5.4 times higher odds of decline in Activities of Daily Living and 6.3 times higher odds of decline in Instrumental Activities of Daily Living that continued two years post-surgery [23].
Frailty likewise impacts discharge patterns. Hospital data indicates non-frail patients are discharged home at twice the rate of frail counterparts (28% versus 14%) [21]. Accordingly, frail patients experience longer hospital stays (7.1 days versus 2.4 days) [22] and require more post-discharge support.
Quality of life measurements reflect this functional disparity. Research utilizing the EQ-5D Index demonstrates higher quality of life correlates with greater life-space mobility (0.02 per life-space assessment score) and decreasing frailty (-0.1 per SD) [9]. Interestingly, individuals with highest frailty levels show twice the quality of life improvement associated with increased life-space mobility than those with lower frailty [9].
Case studies from recent trials
Recent clinical trials provide compelling evidence for frailty assessment’s value. The SPRINT trial revealed that changes in frailty status occur frequently—8.7% of initially robust participants progressed to frailty, while 8.4% of frail participants reversed to robust status within 12 months [5]. Crucially, this study found that neither baseline frailty nor changes in frailty altered the benefit of intensive blood pressure control, reassuring clinicians that frail patients still benefit from guideline-directed therapy [5].
In oncology, a comprehensive review established that frailty assessment predicts mortality (hazard ratio = 1.68), treatment toxicity (odds ratio = 1.83), treatment intolerance (odds ratio = 1.68), and hospitalization risk (odds ratio = 1.94) [24]. Furthermore, the Fitness and Nutrition Program for Seniors (FANS) trial documented improvements in frailty status, health-related quality of life, and fall efficacy following intervention, demonstrating frailty’s modifiable nature when properly identified [25].
Multiple studies confirm that frailty assessment tools like the Clinical Frailty Scale provide high predictive value. In hypertensive outpatients, the CFS achieved 91% negative predictive value for functional decline, helping identify low-risk patients who could tolerate standard treatments [26].
Limitations of Current Frailty Assessment Tools
Despite proven benefits in clinical practice, frailty assessment tools present notable implementation challenges. Current instruments carry inherent limitations that affect their accuracy, usability, and adoption across healthcare settings.
Subjectivity in Clinical Frailty Scale scoring
The Clinical Frailty Scale (CFS), among the most widely used frailty measures, requires substantial clinical judgment that introduces variability between raters. Studies demonstrate CFS scores can vary by as much as two levels between different clinicians assessing the same patient [27]. Analysis of this variation revealed that merely 33.1% of score differences stemmed from actual patient characteristics, while 15.4% resulted from inter-rater differences [27]. The remaining 51.5% variance originated from non-frailty factors such as acute illness severity [27].
Beyond reliability concerns, the CFS may introduce unintended biases. The tool’s heavy emphasis on patient function can create what researchers term an “ableist bias” [10]. For example, patients with congenital or traumatic amputations might receive higher frailty scores than their physiological status warrants [10]. This becomes particularly problematic when these scores influence resource allocation decisions.
Time constraints in acute care settings
Acute care environments present unique barriers to comprehensive frailty evaluation. Neither Comprehensive Geriatric Assessment nor Emergency Geriatric Assessment proves suitable for fast-paced emergency department triage because both are time-consuming and require information rarely available during initial intake [28].
Even seemingly quick tools like the CFS demand more time than typically available. As one study noted, “Although the CFS is touted as a quick and easy test, it does require data collection beyond that which could be collected by a cursory evaluation” [10]. This includes assessing mobility capabilities, habitual physical activity, and functional abilities that emergency settings rarely accommodate [10].
Alongside time pressures, healthcare providers report lacking necessary training. Survey data revealed that many staff cited “a lack of dedicated teaching on frailty screening” and “unclear management pathways for subacute frailty issues” as barriers to implementation [29].
Lack of standardization across tools
Currently, healthcare professionals face a bewildering array of assessment options with no consensus on optimal measurement. Researchers identified at least 60 different frailty measurement tools, with nine in regular use [30]. This variety creates confusion regarding which instrument to select for specific clinical scenarios.
Tools vary substantially in their:
-
Domains assessed (physical, cognitive, social)
-
Implementation method (clinician-administered vs. self-reported)
-
Time requirements (from 3-5 minutes for Edmonton Frail Scale to much longer for Frailty Index) [31]
-
Setting-specific validation (many validated only for particular populations) [31]
The absence of a recognized gold standard further complicates clinical implementation [12]. As one study concluded, “translation from research to clinical practice remains a challenge” [12]. Ultimately, this lack of standardization hinders both comparative research and routine clinical adoption, creating an unfortunate gap between the recognized importance of frailty assessment and its practical implementation.
Barriers to Implementation in Clinical Practice
Despite strong evidence supporting frailty assessment’s value, healthcare organizations face formidable hurdles when attempting systematic implementation. These practical obstacles often determine whether theoretical benefits translate into actual clinical improvements for older adults at risk.
Training gaps among healthcare providers
Research reveals a concerning knowledge deficit among medical professionals regarding frailty concepts and measurement tools. Studies demonstrate that many clinicians struggle with basic frailty definitions and lack awareness of available screening instruments [13]. These gaps ultimately result in neglected assessment and management opportunities in everyday practice.
The education shortage appears widespread, as merely 44% of healthcare providers report receiving any frailty-related training, with only 14% experiencing instruction specifically focused on frailty concepts [32]. Among those with some education, 56% obtained their knowledge through informal “on-the-job” learning, 52% through conferences, and 40% via workplace-based education [32]. Even after receiving training, most practitioners characterize their preparation as only “fairly adequate” (42%) or “somewhat adequate” (31%) [32].
Tool selection confusion: CFS vs FI vs FRAIL
Clinicians face a bewildering array of assessment options without clear guidance on appropriate selection. Various tools likely identify distinct frailty constructs, yet exhibit poor agreement between instruments [33]. This uncertainty regarding which measurement to employ in specific contexts constitutes a major barrier reported by practitioners [32]. Within emergency and critical care environments, the Clinical Frailty Scale has emerged as a frequently studied approach [33]. Meanwhile, modified versions of the Frailty Index model have been recommended for acute care settings [33]. For certain specialized assessments like the Hospital Frailty Risk Score, developers aim to minimize inter-rater bias errors inherent in rapid assessment tools [33]. Nonetheless, without consensus on optimal tool selection, implementation remains fragmented across healthcare settings.
Integration challenges with EHR systems
Electronic health record integration presents additional complications for systematic frailty assessment. Although EHRs provide robust clinical data beyond what’s available in claims, they introduce inconsistencies across providers and health systems [2]. Implementation complexity increases dramatically outside unified health systems like the National Health Service [34].
Data limitations create practical obstacles, with studies showing approximately 15-30% of adults in Accountable Care Organizations couldn’t have electronic frailty indexes calculated due to missing information [34]. Healthier individuals with minimal health system contact often get excluded from such calculations, potentially skewing population-level assessments [34].
Encouragingly, national trends toward increased EHR data harmonization through certification requirements may facilitate greater adoption of electronic frailty indices across different provider platforms [2].
Recommendations for Integrating Frailty Assessment
Integrating frailty assessment into everyday clinical workflows requires practical approaches that balance thoroughness with efficiency. Several healthcare systems have successfully implemented streamlined processes that identify at-risk patients without overburdening practitioners.
Use of rapid screening tools in primary care
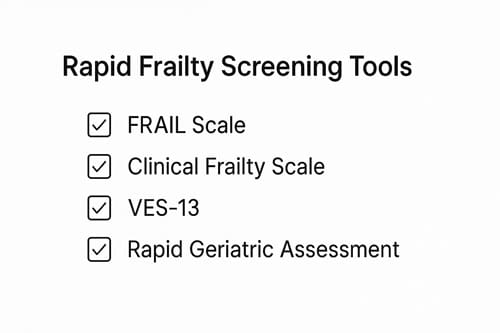
Primary care settings serve as ideal entry points for frailty detection, yet time constraints necessitate brief, validated instruments. The FRAIL scale represents one such option, requiring merely 15-30 seconds to complete while effectively predicting disability and mortality at 9 years [35]. This five-item questionnaire performs comparably to more complex measures, prompting its adoption in Australian and Brazilian primary care guidelines [36]. Equally effective, the Clinical Frailty Scale utilizes pictorial versions with corresponding text to facilitate rapid assessment [36].
For practices seeking alternative options, several validated instruments offer similar benefits:
-
Vulnerable Elders Survey-13 (VES-13): staff or self-administered in under 5 minutes [36]
-
Kihon checklist: 25 yes/no questions evaluating multiple domains [36]
-
Rapid Geriatric Assessment (RGA): screens for frailty, sarcopenia, appetite, and cognition in under 5 minutes [37]
Embedding frailty score in routine checkups
Successfully integrating frailty assessment requires strategic incorporation into existing workflows. Medicare Annual Wellness Visits present natural opportunities to introduce screening without scheduling additional appointments [38]. Throughout routine encounters, practitioners should remain vigilant for telltale indicators that warrant deeper assessment—immobility, incontinence, multiple falls, cognitive decline, and medication side effects should “ring a bell” suggesting possible frailty [35].
Presently, Dutch hospitals demonstrate systematic implementation wherein nurses screen all admitted older adults using tools like the Groningen Frailty Indicator or Maastricht Frailty Screening Tool [39].
Referral pathways for CGA based on frailty level
Effective systems establish clear pathways from screening to intervention. In some hospitals, electronic nursing systems automatically generate specialist consultation orders based on frailty scores or specific risk items [39]. The International Conference on Frailty and Sarcopenia Research Guidelines subsequently recommend comprehensive geriatric assessment following positive initial screens [33].
These structured referral processes recognize frailty assessment as merely the first step. The results serve as starting points for comprehensive evaluation [39], enabling care providers to implement personalized interventions targeting physical activity with resistance components and addressing polypharmacy [40], ultimately transforming screening into meaningful clinical action.

Conclusion 
Frailty assessment serves as a cornerstone of effective geriatric care, yet remains critically underutilized despite its proven benefits. Throughout healthcare settings, skipping this essential evaluation creates ripple effects that compromise patient outcomes, increase system costs, and miss opportunities for early intervention. Clinicians who incorporate these assessments gain valuable insights beyond what chronological age alone can provide, allowing for more nuanced clinical decision-making and personalized care planning.
The consequences of overlooking frailty status extend far beyond immediate patient encounters. Hospital readmissions rise substantially among unidentified frail patients, particularly after the first week post-discharge when the gap between frail and non-frail readmission rates widens dramatically. Additionally, delayed diagnoses of geriatric syndromes occur with greater frequency when frailty goes unrecognized, creating care inequalities throughout the acute care system. Perhaps most concerning, healthcare teams miss crucial early intervention opportunities that could otherwise prevent functional decline.
Financial implications of unassessed frailty burden healthcare systems through extended hospital stays, higher complication rates, and inefficient resource allocation. Frail patients typically require lengthier hospitalization—averaging 20 days compared to 12 days for non-frail individuals—while generating healthcare costs sometimes double those of their non-frail counterparts. These economic realities underscore the pragmatic value of systematic frailty screening beyond its clinical benefits.
Surgical decision-making particularly suffers without proper risk stratification tools. Undoubtedly, frail patients face elevated mortality risks even after minor procedures, yet without appropriate assessment, clinicians cannot identify these high-risk individuals. Consequently, prehabilitation planning opportunities vanish, and both over-treatment and under-treatment scenarios become more common.
Research consistently demonstrates improved outcomes when frailty assessment guides clinical care. Healthcare systems implementing structured screening initiatives have witnessed measurable reductions in mortality and complication rates. Furthermore, recent trials confirm frailty’s modifiable nature when properly identified, offering hope that appropriate interventions can actually reverse frailty status in some patients.
Current assessment tools certainly present limitations—subjective scoring variability, time constraints in acute settings, and lack of standardization create implementation challenges. Nevertheless, these obstacles pale compared to the documented harms of proceeding without any frailty evaluation. Though barriers exist, including training gaps and electronic health record integration difficulties, feasible solutions have emerged.
Moving forward, healthcare practitioners should consider rapid screening tools like the FRAIL scale or Clinical Frailty Scale for efficient assessment. Embedding these evaluations within routine checkups—particularly during Medicare Annual Wellness Visits—creates natural opportunities for frailty detection without imposing excessive burdens on clinical workflows. Clear referral pathways for comprehensive geriatric assessment based on frailty levels then transform screening results into meaningful clinical action.
Ultimately, frailty assessment represents not merely an academic exercise but a fundamental component of patient-centered care. Though implementation challenges exist, the substantial benefits for patient outcomes, resource allocation, and healthcare quality justify the effort required to overcome these barriers. Therefore, healthcare systems and individual practitioners must prioritize frailty assessment as an essential component of comprehensive geriatric care.
Key Takeaways
Healthcare systems face substantial clinical and economic consequences when they skip frailty assessment, despite its proven ability to predict adverse outcomes and guide personalized care decisions.
• Frailty predicts outcomes better than age alone: Frail patients face 3.49 times higher mortality risk and longer hospital stays, making biological age more predictive than chronological age.
• Skipping assessment costs healthcare systems billions: Unrecognized frailty leads to 24% readmission rates versus 14% for non-frail patients, contributing to $15-20 billion in annual readmission costs.
• Surgical risk stratification fails without frailty scores: Even minor procedures carry 10% mortality risk in very frail patients, yet clinicians miss prehabilitation opportunities without proper assessment.
• Simple screening tools enable rapid implementation: The FRAIL scale takes just 15-30 seconds to complete and can be embedded in routine checkups like Medicare Annual Wellness Visits.
• Early identification enables reversible interventions: Studies show 8.4% of frail patients can return to robust status within 12 months when properly identified and treated.
The evidence is clear: implementing systematic frailty assessment transforms theoretical benefits into measurable improvements in patient outcomes, cost reduction, and quality of care across all healthcare settings.
Frequently Asked Questions:
FAQs
Q1. What is considered the most effective method for assessing frailty? The Comprehensive Geriatric Assessment (CGA) is widely regarded as the gold standard for evaluating and managing frailty in older adults. It provides a thorough evaluation of an individual’s physical, cognitive, and functional status.
Q2. Why is it crucial to avoid hospital admissions for frail elderly individuals? Hospital admissions can be particularly risky for frail older adults. Studies suggest that hospitalization may lead to a decline in physical abilities and increase the risk of acquiring infections, which can result in serious complications or even death in this vulnerable population.
Q3. How does frailty assessment benefit patient care? Frailty assessment is crucial for identifying older adults at higher risk of adverse health outcomes. It helps predict increased mortality risk, disability, worsening mobility, falls, and hospitalization, allowing healthcare providers to implement targeted interventions and personalized care plans.
Q4. What are some limitations of current frailty assessment tools? Current frailty assessment tools face challenges in accuracy and specificity. While some tests like slow gait speed and the timed get-up-and-go test have high sensitivity, they often produce false-positive results. This means they cannot be relied upon as single, definitive tests for identifying frailty.
Q5. How can healthcare systems integrate frailty assessment into routine care? Healthcare systems can integrate frailty assessment by using rapid screening tools like the FRAIL scale during routine checkups, such as Medicare Annual Wellness Visits. Embedding these assessments in existing workflows and establishing clear referral pathways for comprehensive geriatric assessment based on frailty levels can help make frailty screening a standard part of care for older adults.
References:
[1] – https://pubmed.ncbi.nlm.nih.gov/31297511/
[2] – https://aspe.hhs.gov/sites/default/files/documents/
0db5e9c6f83a798f4518aee6991930ba/Frailty-EHR-Implementation-Guide-ASPE-RAND.pdf
[3] – https://jamanetwork.com/journals/jamasurgery/fullarticle/2755273
[4] – https://www.sciencedirect.com/science/article/pii/S0197457223002690
[5] – https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-025-04382-7
[6] – https://www.healthaffairs.org/doi/abs/10.1377/hlthaff.2021.00401
[7] – https://pmc.ncbi.nlm.nih.gov/articles/PMC8301636/
[8] – https://pmc.ncbi.nlm.nih.gov/articles/PMC12291383/
[9] – https://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-022-03355-2
[10] – https://www.ncbi.nlm.nih.gov/books/NBK559009/
[11] – https://jamanetwork.com/journals/jamasurgery/fullarticle/2801764
[12] – https://www.frontiersin.org/journals/public-health/articles/10.3389/fpubh.2024.1424613/full
[13] – https://pmc.ncbi.nlm.nih.gov/articles/PMC10084012/
[14] – https://www.sciencedirect.com/science/article/pii/S1279770723000799
[15] – https://pmc.ncbi.nlm.nih.gov/articles/PMC7370792/
[16] – https://pmc.ncbi.nlm.nih.gov/articles/PMC9167908/
[17] – https://www.facs.org/media/xoklzbuy/surgical-palliative-care-frailty-assessment.pdf
[18] – https://pmc.ncbi.nlm.nih.gov/articles/PMC10125297/
[19] – https://jamanetwork.com/journals/jamasurgery/fullarticle/2773447
[20] – https://emj.bmj.com/content/41/9/514
[21] – https://pmc.ncbi.nlm.nih.gov/articles/PMC8600066/
[22] – https://www.jvascsurg.org/article/S0741-5214(20)30696-0/fulltext
[23] – https://www.sciencedirect.com/science/article/pii/S0741521422022005
[24] – https://academic.oup.com/jnci/article/117/7/1316/7991298
[25] – https://pubmed.ncbi.nlm.nih.gov/40552877/
[26] – https://www.sciencedirect.com/science/article/pii/S0953620524002164
[27] – https://academic.oup.com/aging/article/54/4/afaf093/8116546
[28] – https://www.sciencedirect.com/science/article/abs/pii/S0197457219302617
[29] – https://www.jenonline.org/article/S0099-1767(23)00233-7/fulltext
[30] – https://pmc.ncbi.nlm.nih.gov/articles/PMC10288734/
[31] – https://pmc.ncbi.nlm.nih.gov/articles/PMC10883846/
[32] – https://onlinelibrary.wiley.com/doi/10.1111/ajag.13232
[33] – https://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-021-02722-9
[34] – https://pmc.ncbi.nlm.nih.gov/articles/PMC6777083/
[35] – https://pmc.ncbi.nlm.nih.gov/articles/PMC7155305/
[36] – https://pmc.ncbi.nlm.nih.gov/articles/PMC7568453/
[37] – https://www.frontiersin.org/journals/medicine/articles/10.3389/
fmed.2020.00261/full
[38] – https://www.aafp.org/pubs/afp/issues/2021/0215/p219.html
[39] – https://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-021-02586-z
[40] – https://pmc.ncbi.nlm.nih.gov/articles/PMC12186596/

