The Hayflick Limit Exposed: Why Your Diet May Be Setting Your Biological Clock
Please like and subscribe if you enjoyed this video 🙂
Relevant Medical Specialties
1. Geriatrics
-
Core focus on cellular aging, telomere biology, and lifespan modulation
-
Practical insights for preventing age-related decline and enhancing healthspan
2. Internal Medicine (Generalists)
-
Broad applicability to chronic disease prevention
-
Highlights how nutrition and cellular aging intersect with systemic health
3. Endocrinology
-
Telomere dynamics are tightly linked to metabolism, obesity, insulin resistance, and diabetes
-
Diet and oxidative stress are key endocrine concerns
4. Cardiology
-
Telomere shortening and inflammation play key roles in atherosclerosis and cardiovascular aging
-
Lifestyle interventions linked to cardiovascular biomarkers
5. Preventive Medicine / Lifestyle Medicine
-
A direct fit: integrates diet, exercise, stress management, and aging biology
-
Ideal for patient education and health optimization
6. Oncology
-
Discussion of telomerase activity in cancer cells is central to tumor biology
-
Relevant for understanding cancer cell immortality and aging defense mechanisms
7. Family Medicine
-
Provides clinicians with education tools for long-term patient counseling on aging, diet, and wellness
8. Nutrition / Clinical Nutrition
-
Directly tied to nutrient impact on telomere length, DNA methylation, and oxidative stress
-
Offers biochemical mechanisms that deepen nutritional counseling
9. Hematology / Immunology
-
Telomere dynamics and replicative senescence affect immune cell aging and bone marrow function
-
Important for understanding immune senescence and chronic inflammatory states
10. Neurology / Psychiatry
-
Telomere shortening is implicated in neurodegenerative diseases and cognitive decline
-
Chronic stress and mental health affect aging at the cellular level
Introduction
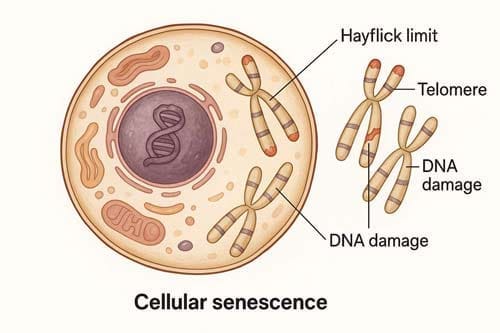
In 1962, the hayflick limit emerged as a revolutionary concept in cell biology when Leonard Hayflick discovered that human cells could only divide a finite number of times before cellular senescence occurs. This groundbreaking theory places the maximum potential human lifespan at approximately 120 years. The hayflick limit refers specifically to the ceiling number of cellular divisions possible before a cell permanently stops reproducing and eventually dies.
While genetics accounts for merely 20-30% of an individual’s lifespan, environmental factors and personal behaviors play a substantially larger role in determining how quickly one approaches this biological boundary. Telomeres—those disposable buffers at chromosome ends—become progressively shorter with each cell division until reaching a critical threshold at which point cell division ceases entirely. Furthermore, research indicates that telomere length declines from approximately 11 kilobases at birth to fewer than 4 kilobases in old age. Though the rate of telomere shortening often doesn’t align perfectly with chronological aging, it can be modified through environmental influences. Interestingly, recent studies demonstrate that intensive lifestyle modifications including dietary changes, regular physical activity, and stress reduction through practices like yoga and meditation can significantly increase telomerase activity in peripheral blood mononuclear cells. For instance, populations in Mediterranean countries not only live longer and healthier lives compared to those in other industrialized nations but also exhibit longer telomeres and higher telomerase activity.
This article examines the hayflick limit theory of aging, provides a comprehensive hayflick limit definition, explores its implications in humans, and most importantly, investigates how dietary choices directly influence this fundamental biological clock that ticks within each of our cells.

Understanding the Hayflick Limit in Human Cells
The cellular replication limit was first observed in 1961 when Leonard Hayflick and Paul Moorhead demonstrated that normal human cells undergo a finite number of divisions before entering a state of irreversible growth arrest. This fundamental discovery overturned previous beliefs about cellular immortality and established the groundwork for modern aging research.
What is the Hayflick Limit?
The Hayflick limit refers to the maximum number of times a normal human somatic cell population can divide before cell division stops completely. For human fetal fibroblasts, this ceiling ranges between 40 and 60 divisions in laboratory conditions. After reaching this threshold, cells enter a phase called senescence—characterized by dramatic alterations in cell morphology, gene expression, metabolism, and epigenetic markers. This phenomenon occurs across diverse cell types and has been documented in numerous species, with fascinating correlations to their respective lifespans. For instance, cells from Galapagos turtles, which can live for centuries, divide approximately 110 times before senescing, whereas mouse cells reach senescence after merely 15 divisions.
Moreover, this discovery directly challenged Alexis Carrel’s earlier theory which incorrectly claimed that normal cells possess unlimited replicative potential. Subsequently, Hayflick interpreted his findings as evidence of aging at the cellular level, suggesting a correlation between cellular aging and the overall physical aging of organisms.
Hayflick Limit Theory of Aging
According to this theory, the progressive accumulation of senescent cells throughout the body contributes substantially to the aging process. As these non-dividing cells increase in number, tissue regeneration capacity declines, leading to age-related deterioration. Research suggests an “ultimate Hayflick limit” of approximately 125 years for humans—a biological ceiling beyond which no amount of diet, exercise, or genetic modification against diseases can extend human lifespan.
Nevertheless, questions remain about how directly this cellular phenomenon relates to organismal aging. Laboratory studies indicate that cellular replicative capacity may correlate primarily with species body mass or lifespan rather than chronological age. Indeed, the limited ability of cells to replicate in culture appears directly relevant to the overall physical aging process experienced by organisms.
Cellular Senescence and the End-Replication Problem
At the molecular level, the Hayflick limit stems from a phenomenon known as the “end-replication problem.” During DNA replication, DNA polymerases cannot completely copy the telomere regions at chromosome ends. This occurs because RNA primers, which initiate DNA synthesis during lagging-strand replication, must be removed. Upon removal of the final primer at the 3′ end, the newly synthesized strand inevitably loses nucleotides.
Consequently, with each cell division, telomeres progressively shorten by 50-100 base pairs. Once telomeres reach a critically short length, the protective cap structure at chromosome ends becomes disrupted. The cell recognizes these uncapped telomeres as DNA double-strand breaks, triggering a persistent DNA damage response that halts further division. This protective mechanism prevents cells with potentially dangerous genetic abnormalities from continuing to replicate.
Interestingly, cancer cells evade the Hayflick limit through the expression of telomerase—an enzyme that extends telomeres and prevents their shortening. This enzymatic activity essentially grants cancer cells unlimited replicative potential, highlighting why Hayflick was the first to observe that predominantly cancer cells demonstrate immortality.
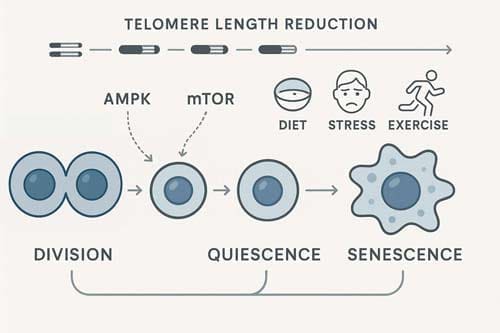
Telomeres, Telomerase, and the Biological Clock
Telomeres serve as protective caps at chromosome ends, functioning as a biological clock that determines cellular lifespan within the framework of the hayflick limit. These specialized nucleoprotein structures prevent chromosomes from nucleolytic degradation, unnecessary recombination, and interchromosomal fusion. As cells divide, telomeres progressively shorten by 50-200 base pairs per division due to the inability of DNA polymerase to completely replicate the lagging strand—a phenomenon known as the “end-replication problem”.
Telomere Shortening and DNA Damage Response
When telomeres reach a critical length, the cell activates a DNA damage response (DDR), triggering cellular senescence or apoptosis. This biological mechanism helps explain why cells hit the hayflick limit after a predetermined number of divisions. Critically short telomeres lose their protective function, becoming largely devoid of telomeric DNA and telomere-binding proteins. At this point, they’re recognized as DNA double-strand breaks, activating sensor kinases (ATM/ATR, DNA-PK) and forming DNA damage foci containing phosphorylated histone H2A.X (γH2A.X).
Interestingly, telomeric regions are particularly susceptible to oxidative damage. The G-rich telomere repeat sequence is highly vulnerable to oxidative stress, which can directly damage telomeres and accelerate shortening beyond what occurs through normal replication. Unlike damage elsewhere in the genome, DNA damage at telomeres is resistant to repair and therefore accumulates in cells, creating telomere-associated foci (TAFs) that persist even in non-dividing cells. Hence, both replicative senescence stimulated by critically short telomeres and senescence-like conditions induced by broken telomeres in non-replicating cells share continuous telomere DDR activation as their common underlying cause.
Telomerase Activation in Stem vs Somatic Cells
Telomerase, a ribonucleoprotein complex consisting of telomerase reverse transcriptase (TERT) and telomerase RNA component (TERC), counteracts telomere shortening by adding telomeric repeats to chromosome ends. This enzyme’s activity is strictly regulated in a cell-type dependent manner. In humans, telomerase is active during early in-utero development, becomes inactivated in most adult somatic cells, yet remains active in germ line, embryonic stem cells, and certain immune cells.
Adult stem cells maintain lower levels of telomerase activity than embryonic stem cells—sufficient to slow telomere shortening and expand replicative lifespan, yet inadequate to prevent eventual replicative senescence. Additionally, telomerase components exhibit dynamic behavior within cells. In HeLa cells, TERT and TERC accumulate in separate intranuclear sites during most of the cell cycle, only being recruited to telomeres during S phase—peaking in the middle of this phase.
Telomere Stability vs Telomere Length
Contrary to common belief, telomere function depends not only on length but importantly on structural integrity. Persistent DNA damage foci can form at telomeres regardless of their length, as demonstrated in studies of mouse tissues where TAFs increase with age despite mice having long telomeres and active telomerase. In fact, these studies revealed no statistically significant difference between telomere length distributions in TAFs versus non-TAFs in aged mice intestinal crypts.
The shelterin complex—consisting of six proteins including TRF1, TRF2, and POT1—plays a critical role in telomere protection. TRF2 deletion in normal somatic cells triggers remarkable phenotypes including rampant telomere fusions, robust activation of ATM-dependent DNA damage signaling, and irreversible cell cycle arrest or cell death. Beyond protection, shelterin components assist in telomere replication, with TRF1 deletion severely impacting this process and resulting in frequent fork stalling and telomere abnormalities.
Ultimately, telomere stability can be compromised through multiple mechanisms, including oxidative damage, replication stress, and inadequate protection by telomere-binding proteins—all contributing to the finite replicative capacity defined by the hayflick limit theory of aging.
How Diet Influences Telomere Dynamics
Dietary patterns exert profound effects on cellular aging through multiple pathways that directly impact telomere maintenance and the hayflick limit. Recent research demonstrates that nutritional choices can accelerate or delay the pace at which cells approach their finite replicative capacity.
Oxidative Stress from Poor Diet Choices
The G-rich sequence of telomeres (TTAGGG) makes them particularly vulnerable targets for reactive oxygen species (ROS) damage. Oxidative stress creates 8-oxo-2′-deoxyguanosine (8-oxodG) within cells, causing DNA double-strand breaks specifically at the GGG sequences of telomeres. Diets high in processed meats, carbohydrates, animal products, and high-fat content generate excessive ROS that attack these susceptible regions.
Excessive sugar consumption initiates a particularly damaging cycle. High intake of sugar and sweetened beverages elevates blood glucose levels, triggering oxidative stress in endothelial cells—a mechanism similar to what occurs in diabetes mellitus. This metabolic disruption has been observed to accelerate telomere shortening. Meanwhile, ROS produced by dysfunctional mitochondria can directly damage telomeric DNA, triggering DNA damage response pathways and ultimately causing cellular senescence.
Inflammation and Telomere Attrition
A bidirectional relationship exists between inflammation and telomere dysfunction. Pro-inflammatory cytokines like TNF-α and IL-6 are elevated in chronic inflammatory conditions and have been found to inhibit telomerase activity, particularly the catalytic subunit TERT. Simultaneously, cells with critically short telomeres often enter senescence and develop a “senescence-associated secretory phenotype” (SASP), releasing inflammatory molecules that perpetuate a damaging feedback loop.
This creates what researchers describe as a “circulus vitiosus” where telomere dysfunction increases inflammatory signaling, which further accelerates telomere attrition. Chronic inflammation aggravates telomere dysfunction and cell senescence while decreasing regenerative potential in multiple tissues. Even low-grade chronic inflammation, common in poor dietary patterns, can directly cause ROS-mediated telomeric DNA damage.
Dietary Choices and the Hayflick Limit
Certain dietary patterns show promising effects on extending cellular lifespan before reaching the hayflick limit. The Mediterranean diet has been associated with longer telomeres and higher telomerase activity in peripheral blood mononuclear cells. In contrast, Western diets accelerate telomere shortening, pushing cells toward their hayflick limit prematurely.
Foods that positively influence telomere dynamics include:
- Fish, nuts, seeds, and olive oil (rich in omega-3 fatty acids)
- Colored fruits and cruciferous vegetables (high in antioxidants)
- Green tea, red grapes, and tomatoes (containing polyphenols)
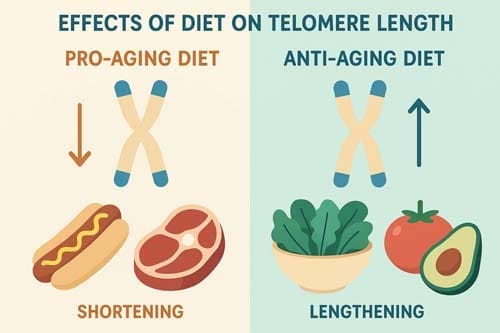
Conversely, dairy products, simple sugars, and foods with high glycemic load are associated with telomere shortening. Through these mechanisms, dietary choices directly influence how quickly cells approach the hayflick limit—the theoretical ceiling of cellular divisions before senescence occurs.
Nutrients That Support Telomere Maintenance
Specific micronutrients play crucial roles in maintaining telomere integrity and potentially delaying the hayflick limit through distinct biochemical mechanisms. Their strategic consumption may influence how rapidly cells approach their finite division capacity.
Folate and Vitamin B12 in DNA Methylation
Folate and vitamin B12 function as essential cofactors in DNA methylation and nucleotide synthesis pathways. These B vitamins are required for converting homocysteine to methionine, which subsequently forms S-adenosyl methionine (SAM)—the primary methyl donor for DNA methylation. When deficient, homocysteine levels rise, correlating with shorter telomeres in cross-sectional human studies. This association exists primarily because homocysteine accumulation increases oxidative stress and inflammation, accelerating telomere attrition. Additionally, folate deficiency causes excessive uracil misincorporation into DNA, generating abasic sites and potentially unrepaired single-strand breaks at telomeres.
Vitamin C, D, and E as Antioxidants
Vitamin C serves as a potent free radical scavenger that protects telomeres from oxidative damage. Studies demonstrate that vitamin C intake positively correlates with leukocyte telomere length and can reduce telomere shortening by up to 62% in cultured human blood vessel cells. Likewise, vitamin D supplementation (2000 IU daily) increases telomerase activity by more than 19%. A recent clinical trial showed that daily vitamin D3 supplementation significantly reduced telomere attrition over four years, preserving approximately 140 base pairs compared to placebo—effectively preventing nearly three years of biological aging. Vitamin E, available in eight different forms, dramatically slows age-related telomere shortening even when exposed to powerful oxidants like hydrogen peroxide.
Polyphenols: Resveratrol and Curcumin
Polyphenols represent a vast group of plant compounds with over 8,000 identified molecules that enhance cellular antioxidant protection. Resveratrol exerts anti-senescent effects on endothelial progenitor cells by enhancing telomerase function and Akt phosphorylation. At merely 5 nmol/L concentration, resveratrol significantly reverses senescence markers in later-passage mesenchymal stem cells through upregulation of hTERT mRNA. Alternatively, curcumin inhibits telomerase action in tumor cells and induces telomere shortening, indicating its potential anticancer properties.
Omega-3 Fatty Acids and Telomere Preservation
Omega-3 fatty acids preserve telomere length through anti-inflammatory mechanisms. Research confirms that reducing plasma omega-6 levels while increasing omega-3s results in longer telomeres. A meta-analysis of five clinical trials involving 337 participants revealed a statistically significant positive effect of omega-3 supplementation on telomere length. Furthermore, omega-3 fatty acids reduce F2-isoprostanes and potentially increase clearance of neutrophils with shorter telomeres from circulation.
Dietary Patterns and Their Impact on Aging
Beyond individual nutrients, comprehensive dietary patterns exert distinct influences on cellular aging mechanisms and potentially alter how quickly cells approach the hayflick limit—the theoretical ceiling for cellular divisions.
Mediterranean Diet and Telomerase Activity
The Mediterranean diet demonstrates remarkable effects on telomere dynamics. Individuals adhering to this dietary pattern exhibit longer leukocyte telomeres and greater telomerase activity in peripheral blood mononuclear cells. Even a short-term (approximately one month) Mediterranean diet intervention reduces the proportion of endothelial cells with shorter telomeres. Notably, higher adherence to the Mediterranean diet correlates with telomere length z-scores of 0.072 versus -0.038 for those with lower adherence. This difference corresponds roughly to 1.5 years of aging per one-point change in Mediterranean diet score, with a three-point change equivalent to approximately 4.5 years of biological aging. Primarily, the diet’s protective mechanisms stem from its rich phytochemical content, which simultaneously reduces oxidative damage and inflammation.
Western Diet and Accelerated Telomere Loss
Alternatively, the Western diet—characterized by high intake of sugars, saturated fats, and processed foods—accelerates telomere attrition through multiple pathways. This dietary pattern promotes hypercaloric intake that generates reactive oxygen species and induces endoplasmic reticulum stress. Among mice bearing tumors, Western diet consumption activated unfolded protein response pathways and enhanced tumor growth. Furthermore, this diet creates persistent low-grade inflammation or “inflammaging,” which contributes to cancer induction and progression.
Intermittent Fasting and Cellular Repair
Intermittent fasting presents another approach for managing cellular aging processes. This dietary strategy triggers a “metabolic switch” where ketone bodies (particularly β-hydroxybutyrate) serve as alternative fuel sources. Among its multiple benefits, fasting activates fatty acid oxidation, enhances intestinal stem cell function, reduces oxidative damage, and improves mitochondrial function. Clinical studies have documented improvements in cardiovascular markers, blood pressure, and enhanced cognitive functions. The molecular mechanisms involve regulation of AMP-activated protein kinase, sirtuins, and mTOR pathways—all linked to longevity genes.
Vegan and Ketogenic Diets: Pros and Cons
Both vegan and ketogenic diets offer distinct advantages regarding cellular aging. Vegan diets correlate with a 75% lower risk of developing hypertension and up to 78% reduced risk of type 2 diabetes. Throughout 18-week interventions, participants following vegan diets lost approximately 5.5 pounds more than non-vegetarians. Similarly, ketogenic diets show effectiveness for weight management, blood sugar control, and cardiovascular risk reduction. However, their combination presents challenges yet potential benefits—the vegan keto approach emphasizes high-fat plant foods like coconut oil, avocados, nuts, and seeds while maintaining ketosis. Although no studies specifically examine telomere impacts of combined vegan-keto approaches, each separately influences pathways affecting cellular senescence.

Conclusion
The research presented throughout this article demonstrates the profound connection between dietary choices and cellular aging processes. Telomere maintenance, therefore, emerges as a critical factor in how rapidly cells approach the Hayflick limit. Evidence suggests that diet-induced oxidative stress, inflammation, and nutrient deficiencies directly accelerate telomere shortening, while proper nutritional choices can delay this process substantially.
Mediterranean dietary patterns stand out as particularly beneficial for telomere preservation, extending cellular lifespan through reduced oxidation and inflammation. This contrasts sharply with Western diets that promote telomere attrition through multiple pathways. Certain micronutrients—namely folate, vitamins C, D, E, and omega-3 fatty acids—play essential roles in telomere maintenance through distinct biochemical mechanisms including DNA methylation, antioxidant protection, and anti-inflammatory effects.
Alternative eating patterns such as intermittent fasting trigger cellular repair mechanisms that potentially delay senescence. Both vegan and ketogenic approaches offer unique advantages regarding age-related cellular changes, though each comes with distinct considerations for long-term adherence.
The ultimate implications extend beyond mere cellular longevity. Telomere preservation affects overall organismal aging, disease susceptibility, and quality of life. Although genetics contribute 20-30% to individual lifespan, dietary choices represent modifiable factors with substantial impact on biological aging trajectories. These findings underscore the remarkable plasticity of the aging process at the cellular level despite the theoretical ceiling imposed by the Hayflick limit.
Future research will undoubtedly refine our understanding of specific dietary interventions that optimize telomere maintenance. Until then, evidence strongly supports dietary patterns rich in plant compounds, omega-3 fatty acids, and essential micronutrients while limiting processed foods and excessive sugar consumption. Through these nutritional strategies, individuals may effectively slow their biological clocks, potentially extending both lifespan and healthspan despite the fundamental constraints of cellular replication.
Key Takeaways
Understanding how your dietary choices influence cellular aging can help you make informed decisions to potentially slow your biological clock and extend healthspan within the constraints of the Hayflick limit.
• Your cells have a division limit: Human cells can only divide 40-60 times before entering senescence, setting a biological ceiling of approximately 120 years for human lifespan.
• Diet directly affects telomere health: Poor dietary choices accelerate telomere shortening through oxidative stress and inflammation, while nutrient-rich foods protect these cellular timekeepers.
• Mediterranean diet extends cellular lifespan: This eating pattern increases telomerase activity and preserves telomere length, effectively slowing biological aging by up to 4.5 years.
• Key nutrients support telomere maintenance: Vitamins C, D, E, folate, B12, and omega-3 fatty acids protect against cellular aging through antioxidant and anti-inflammatory mechanisms.
• Western diets accelerate aging: High sugar, processed foods, and saturated fats create oxidative damage that pushes cells toward their division limit prematurely.
While genetics account for only 20-30% of lifespan, your daily food choices represent a powerful tool for influencing how quickly your cells age. By prioritizing nutrient-dense, anti-inflammatory foods and avoiding processed options, you can potentially extend both lifespan and healthspan despite the fundamental biological constraints of cellular replication.
Frequently Asked Questions:
FAQs
Q1. What is the Hayflick limit and how does it relate to aging? The Hayflick limit refers to the maximum number of times human cells can divide before they stop, typically 40-60 times. This cellular phenomenon is linked to aging, as it sets a theoretical maximum human lifespan of about 120 years.
Q2. How does diet influence cellular aging and the Hayflick limit? Diet significantly impacts cellular aging by affecting telomere length and health. Nutrient-rich foods can protect telomeres and slow aging, while poor dietary choices can accelerate telomere shortening, pushing cells closer to their division limit prematurely.
Q3. What dietary pattern is most beneficial for preserving telomeres? The Mediterranean diet has shown remarkable benefits for telomere preservation. It increases telomerase activity and maintains telomere length, potentially slowing biological aging by up to 4.5 years compared to other dietary patterns.
Q4. Which nutrients are crucial for maintaining telomere health? Key nutrients that support telomere maintenance include vitamins C, D, and E, folate, vitamin B12, and omega-3 fatty acids. These nutrients protect against cellular aging through antioxidant and anti-inflammatory mechanisms.
Q5. Can lifestyle changes affect how quickly we approach the Hayflick limit? Yes, lifestyle changes, particularly dietary choices, can significantly influence how quickly cells approach the Hayflick limit. By prioritizing nutrient-dense, anti-inflammatory foods and avoiding processed options, individuals may potentially extend both their lifespan and healthspan within biological constraints.
References:
[1] – https://www.sciencedirect.com/science/article/abs/pii/S1568163723000673
[2] – https://pmc.ncbi.nlm.nih.gov/articles/PMC9570627/
[3] – https://pmc.ncbi.nlm.nih.gov/articles/PMC8189250/
[4] – https://pubmed.ncbi.nlm.nih.gov/26293976/
[5] – https://pmc.ncbi.nlm.nih.gov/articles/PMC3370421/
[6] – https://www.frontiersin.org/journals/genetics/articles/
10.3389/fgene.2020.630186/full
[7] – https://www.cell.com/cell/fulltext/S0092-8674(13)00016-0
[8] – https://pmc.ncbi.nlm.nih.gov/articles/PMC11200901/
[9] – https://www.aginganddisease.org/EN/10.14336/AD.2023.1128
[10] – https://pmc.ncbi.nlm.nih.gov/articles/PMC9598777/
[11] – https://pmc.ncbi.nlm.nih.gov/articles/PMC7411297/
[12] – https://www.sciencedirect.com/science/article/abs/pii/S096289242100266X
[13] – https://www.healthline.com/nutrition/vegan-keto-diet
[14] – https://pmc.ncbi.nlm.nih.gov/articles/PMC5042391/
[15] – https://www.mdpi.com/1422-0067/25/15/8542
[16] – https://www.nature.com/articles/ncomms5172
[17] – https://pmc.ncbi.nlm.nih.gov/articles/PMC4761710/
[18] – https://nyaspubs.onlinelibrary.wiley.com/doi/full/10.1111/j.1749-6632.2011.06101.x
[19] – https://aacrjournals.org/cancerpreventionresearch/
article/7/1/128/50227/Folate-Deficiency-Induces-Dysfunctional-Long-and
[20] – https://pmc.ncbi.nlm.nih.gov/articles/PMC9908946/
[21] – https://www.lifeextension.com/magazine/2016/12/research-update?srsltid=AfmBOooilJcDZzb1OprvLsGm6NG-enysGZJO0cFD_PZ3HvqLFsVDzNvi
[22] – https://news.harvard.edu/gazette/story/2025/05/vitamin-d-supplements-may-slow-biological-aging/
[23] – https://pmc.ncbi.nlm.nih.gov/articles/PMC10740764/
[24] – https://immunityageing.biomedcentral.com/articles/10.1186/s12979-025-00522-y
[25] – https://www.degruyterbrill.com/document/doi/10.1515/bmc-2021-0024/html?lang=en&srsltid=AfmBOoo1MuylOlsNmeo_7tPc8Lo67f95JWvyAFgCPlENteUahaq6qqFV
[26] – https://www.bmj.com/content/349/bmj.g6674
[27] – https://www.nature.com/articles/s41419-021-03929-9
[28] – https://pmc.ncbi.nlm.nih.gov/articles/PMC11262604/
