Vyepti
2.1 Recommended Dosing
The recommended dosage is 100 mg administered by intravenous infusion every 3 months. Some patients may benefit from a dosage of 300 mg administered by intravenous infusion every 3 months.
2.2 Dilution Instructions
VYEPTI requires dilution prior to administration. Dilute only in 100 mL 0.9% Sodium Chloride Injection, USP. The infusion bags must be made of polyvinyl chloride (PVC), polyethylene (PE), or polyolefin (PO). Use appropriate aseptic technique when preparing VYEPTI solution for intravenous infusion. VYEPTI single-dose vials contain no preservative; discard unused portion remaining in the vial.
Dilution
100 mg dose:
To prepare the solution, withdraw 1 mL of VYEPTI from a single-dose vial using a sterile needle and syringe. Inject the 1 mL content into a 100 mL bag of 0.9% Sodium Chloride Injection, USP.
300 mg dose:
To prepare the solution, withdraw 1 mL of VYEPTI from each of 3 single-dose vials using a sterile needle and syringe. Inject the resulting 3 mL content into a 100 mL bag of 0.9% Sodium Chloride Injection, USP.
Storage and Handling of Diluted Product
Gently invert the VYEPTI solution to mix completely. Do not shake. Following dilution, VYEPTI solution must be infused within 8 hours. During this time, VYEPTI solution should be stored at room temperature, 20°C to 25°C (68°F to 77°F). Do not freeze.
2.3 Infusion Administration Instructions
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if the liquid contains visible particulate matter or is cloudy or discolored [see Dosage Forms and Strengths (3)].
No other medications should be administered through the infusion set or mixed with VYEPTI. VYEPTI is for intravenous infusion only; infuse over approximately 30 minutes. Do not administer VYEPTI as an intravenous push or bolus injection. Use an intravenous infusion set with a 0.2 micron or 0.22 micron in-line or add-on sterile filter. After the infusion is complete, flush the line with 20 mL of 0.9% Sodium Chloride Injection, USP.
3 DOSAGE FORMS AND STRENGTHS
VYEPTI is a clear to slightly opalescent, colorless to brownish-yellow solution available as follows:
- Injection: 100 mg/mL in a single-dose vial
4 CONTRAINDICATIONS
VYEPTI is contraindicated in patients with serious hypersensitivity to eptinezumab-jjmr or to any of the excipients in VYEPTI. Reactions have included anaphylaxis and angioedema [see Warnings and Precautions (5.1)].
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including angioedema, urticaria, facial flushing, and rash, have occurred with VYEPTI in clinical trials. Most hypersensitivity reactions occurred during infusion and were not serious, but often led to discontinuation or required treatment. Serious hypersensitivity reactions may occur. Cases of anaphylaxis have been reported in the postmarketing setting. If a hypersensitivity reaction occurs, consider discontinuing VYEPTI and institute appropriate therapy [see Contraindications (4) and Patient Counseling Information (17)].
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of VYEPTI was evaluated in 2076 patients with migraine who received at least one dose of VYEPTI, representing 1615 patient-years of exposure; of these, 1524 patients were exposed to 100 mg or 300 mg. Across all doses, 1872 patients were exposed for at least 6 months and 991 patients were exposed for 12 months. In the placebo-controlled clinical studies (Study 1 and Study 2) of 1372 patients, 579 patients received at least one dose of VYEPTI 100 mg, 574 patients received at least one dose of VYEPTI 300 mg, and 588 patients received placebo [see Clinical Studies (14)]. Approximately 86% were female, 89% were white, and the mean age was 40.4 years at study entry.
The most common (incidence at least 2% and at least 2% greater than placebo) adverse reactions in the clinical trials for the preventive treatment of migraine were nasopharyngitis and hypersensitivity.
Table 1 summarizes the adverse reactions that occurred during Study 1 and Study 2.
Table 1. Adverse Reactions Occurring with an Incidence of at Least 2% for VYEPTI and at Least 2% Greater than Placebo in Studies 1 and 2
|
Adverse Reactions |
VYEPTI 100 mg N=579 % |
VYEPTI 300 mg N=574 % |
Placebo N=588 % |
|
Nasopharyngitis |
6 |
8 |
6 |
|
Hypersensitivity reactions* |
1 |
2 |
0 |
* Hypersensitivity reactions includes multiple related adverse event terms, such as hypersensitivity, pruritus, and flushing/hot flush that occurred on the day of dosing.
In Study 1 and Study 2, 1.9% of patients treated with VYEPTI discontinued treatment because of adverse reactions [see Warnings and Precautions (5.1)].
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to eptinezumab-jjmr in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
In patients receiving VYEPTI 100 mg or 300 mg every 3 months, the incidence of anti-eptinezumab-jjmr antibody development in Study 1 (up to 56 weeks) was 20.6% (92/447), and 41.3% (38/92) of those patients developed anti-eptinezumab-jjmr neutralizing antibodies. In Study 2 (up to 32 weeks), the incidence of anti-eptinezumab-jjmr antibody development was 18.3% (129/706), and 34.9% (45/129) of those patients developed anti-eptinezumab-jjmr neutralizing antibodies. In an open-label study with 84 weeks of treatment, 18% (23/128) of patients developed anti-eptinezumab-jjmr antibodies, and 39% (9/23) of those patients developed anti-eptinezumab-jjmr neutralizing antibodies.
Although the results from both studies showed no clear evidence of an impact from development of anti-eptinezumab-jjmr antibodies, including neutralizing antibodies, on the safety and efficacy profiles of VYEPTI, the available data are too limited to make definitive conclusions.
6.3 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of VYEPTI. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders – Anaphylaxis [see Contraindications (4) and Warnings and Precautions (5.1)].
8.1 Pregnancy
Risk Summary
There are no adequate data on developmental risks associated with the use of VYEPTI in pregnant women.
No adverse developmental effects were observed following administration of eptinezumab-jjmr to pregnant animals at doses greater than those used clinically [see Data].
In the U.S. general population, the estimated background risk of major birth defects and miscarriages in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively. The estimated rate of major birth defects (2.2%-2.9%) and miscarriage (17%) among deliveries to women with migraine are similar to rates reported in women without migraine.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Published data have suggested that women with migraine may be at increased risk of preeclampsia and gestational hypertension during pregnancy.
Data
Animal Data
When eptinezumab-jjmr (0, 75, or 150 mg/kg) was administered weekly to female rats and rabbits by intravenous injection throughout organogenesis, no adverse effects on embryofetal development were observed. The higher dose tested (150 mg/kg) is 30 times the maximum recommended human dose (MRHD) of 300 mg, on a body weight basis (mg/kg).
When eptinezumab-jjmr (0, 75, or 150 mg/kg) was administered weekly to female rats throughout pregnancy and lactation, no adverse effects on pre- and postnatal development were observed. The higher dose tested (150 mg/kg) is 30 times the MRHD, on a mg/kg basis.
8.2 Lactation
Risk Summary
There are no data on the presence of eptinezumab-jjmr in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VYEPTI and any potential adverse effects on the breastfed infant from VYEPTI or from the underlying maternal condition.
8.5 Geriatric Use
Clinical studies of VYEPTI did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients.
11 DESCRIPTION
Eptinezumab-jjmr is a humanized immunoglobulin G1 (IgG1) monoclonal antibody specific for calcitonin gene-related peptide (CGRP) ligand. Eptinezumab-jjmr has an approximate molecular weight of 143 kD. Eptinezumab-jjmr is produced in Pichia pastoris yeast cells by recombinant DNA technology.
VYEPTI (eptinezumab-jjmr) injection is a sterile, preservative-free, clear to slightly opalescent, colorless to brownish-yellow solution, for intravenous infusion. VYEPTI is supplied as a 100 mg/mL single-dose vial. Each mL contains 100 mg eptinezumab-jjmr formulated in L-histidine (1 mg), L-histidine hydrochloride monohydrate (2.8 mg), polysorbate 80 (0.15 mg), sorbitol (40.5 mg), and Water for Injection, USP, at a pH of 5.8.
12 CLINICAL PHARMACOLOGY
Eptinezumab-jjmr is a humanized monoclonal antibody that binds to calcitonin gene-related peptide (CGRP) ligand and blocks its binding to the receptor.
12.1 Mechanism of Action
Eptinezumab-jjmr is a humanized monoclonal antibody that binds to calcitonin gene-related peptide (CGRP) ligand and blocks its binding to the receptor.
12.2 Pharmacodynamics
The relationship between the pharmacodynamic activity and the mechanism(s) by which eptinezumab-jjmr exerts its clinical effects is unknown.
12.3 Pharmacokinetics
Eptinezumab-jjmr exhibits linear pharmacokinetics and exposure increases proportionally with doses from 100 mg to 300 mg after intravenous administration. Steady-state plasma concentration is attained after the first dose with a once every 3-month dosing schedule.
Distribution
The central volume of distribution (Vc) for eptinezumab-jjmr is approximately 3.7 liters.
Metabolism & Elimination
Eptinezumab-jjmr is expected to be degraded by proteolytic enzymes into small peptides and amino acids.
The apparent clearance of eptinezumab-jjmr was 0.006 L/h, and the terminal elimination half-life was approximately 27 days.
Specific Populations
A population pharmacokinetic analysis assessing the effects of age, race, sex, and body weight did not suggest any clinically significant impact of these covariates on eptinezumab exposures.
Patients with Renal or Hepatic Impairment
No dedicated studies were conducted to assess the effects of renal or hepatic impairment on the pharmacokinetics of eptinezumab-jjmr. However, hepatic or renal impairment is not expected to affect the pharmacokinetics of eptinezumab-jjmr. A population pharmacokinetic analysis of integrated data from eptinezumab-jjmr clinical studies did not reveal clinically significant impact on pharmacokinetics of patients with hepatic or renal impairment.
Drug Interaction Studies
P450 Enzymes
Eptinezumab-jjmr is not metabolized by cytochrome P450 enzymes; therefore, interactions with concomitant medications that are substrates, inducers, or inhibitors of cytochrome P450 enzymes are unlikely.
Sumatriptan
The co-administration of a single dose of 300 mg eptinezumab-jjmr administered as an intravenous infusion (over a period of 1 hour ± 15 min) with a single dose of 6 mg sumatriptan administered subcutaneously did not significantly influence the pharmacokinetics of eptinezumab-jjmr or sumatriptan.
13 NONCLINICAL TOXICOLOGY
Carcinogenesis
The carcinogenic potential of eptinezumab-jjmr has not been assessed.
Mutagenesis
Genetic toxicology studies of eptinezumab-jjmr have not been conducted.
Impairment of Fertility
When eptinezumab-jjmr (0, 75, or 150 mg/kg) was administered weekly by intravenous injection to male and female rats prior to and during mating and continuing in females to gestation day 3-4, no adverse effects on fertility were observed. The higher dose tested (150 mg/kg) is 30 times the maximum recommended human dose of 300 mg, on a body weight basis (mg/kg).
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
The carcinogenic potential of eptinezumab-jjmr has not been assessed.
Mutagenesis
Genetic toxicology studies of eptinezumab-jjmr have not been conducted.
Impairment of Fertility
When eptinezumab-jjmr (0, 75, or 150 mg/kg) was administered weekly by intravenous injection to male and female rats prior to and during mating and continuing in females to gestation day 3-4, no adverse effects on fertility were observed. The higher dose tested (150 mg/kg) is 30 times the maximum recommended human dose of 300 mg, on a body weight basis (mg/kg).
14 CLINICAL STUDIES
The efficacy of VYEPTI was evaluated as a preventive treatment of episodic and chronic migraine in two randomized, multicenter, placebo-controlled studies, both with 6-month double-blind periods: one study in patients with episodic migraine (Study 1) and one study in patients with chronic migraine (Study 2). VYEPTI was administered by intravenous infusion every 3 months in both studies; however, the primary endpoint was measured at 12 weeks.
Study 1: Episodic Migraine
Study 1 (NCT02559895) included adults with a history of episodic migraine (4 to 14 headache days per month, of which at least 4 were migraine days). A total of 665 patients were randomized to receive placebo (N=222), 100 mg VYEPTI (N=221), or 300 mg VYEPTI (N=222) every 3 months for 12 months. Patients were allowed to use concurrent acute migraine or headache medications, including migraine-specific medications (i.e., triptans, ergotamine derivatives), during the trial.
The study excluded patients with a history of cardiovascular disease (hypertension, ischemic heart disease), neurological disease, or cerebrovascular disease.
The primary efficacy endpoint was the change from baseline in mean monthly migraine days over Months 1-3. Secondary endpoints included the percentages of patients with 50% or greater, and 75% or greater reductions from baseline in monthly migraine days over Months 1-3.
Patients had a median age of 39 years (range: 18 to 71 years), 84% were female, and 84% were white. The mean migraine frequency at baseline was approximately 8.6 migraine days per month and was similar across treatment groups.
VYEPTI treatment demonstrated statistically significant improvements compared to placebo for the primary efficacy endpoint, as shown in Table 2; secondary endpoints are also summarized in Table 2.
Table 2. Efficacy Endpoint Results in Study 1
|
VYEPTI 100 mg N=221 |
VYEPTI 300 mg
N=222
|
Placebo
N=222
|
|
| Monthly Migraine Days (MMD) – Months 1-3 |
|
||
| Change from baseline | -3.9 | -4.3 | -3.2 |
| Difference from placebo | -0.7 | -1.1 | |
| p-value | 0.018 | <0.001 | |
| ≥50% MMD responders – Months 1-3 | |||
| % Responders | 49.8% | 56.3% | 37.4% |
| Difference from placebo | 12.4% | 18.9% | |
| p-value | 0.009* |
<0.001 |
|
| ≥75% MMD responders – Months 1-3 | |||
| % Responders | 22.2% | 29.7% | 16.2% |
| Difference from placebo | 6.0% | 13.5% | |
| p-value | NS** | <0.001 | |
* Nominal statistical significance
** NS = Not statistically significant
Figure 1 shows the mean change from baseline in average monthly migraine days in Study 1. Patients treated with VYEPTI at both doses had greater mean decreases from baseline in mean monthly migraine days over Months 1-3 compared to placebo-treated patients.
Figure 1. Change from Baseline in Monthly Migraine Days in Study 1
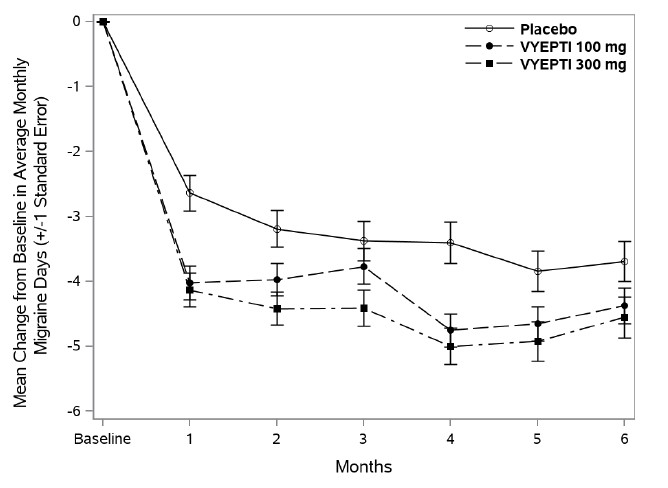
Figure 2 shows the distribution of change from baseline in mean monthly migraine days through Month 3 by treatment group in 2-day increments.
Figure 2. Distribution of Change from Baseline in Mean Monthly Migraine Days over Months 1 to 3 by Treatment Group in Study 1
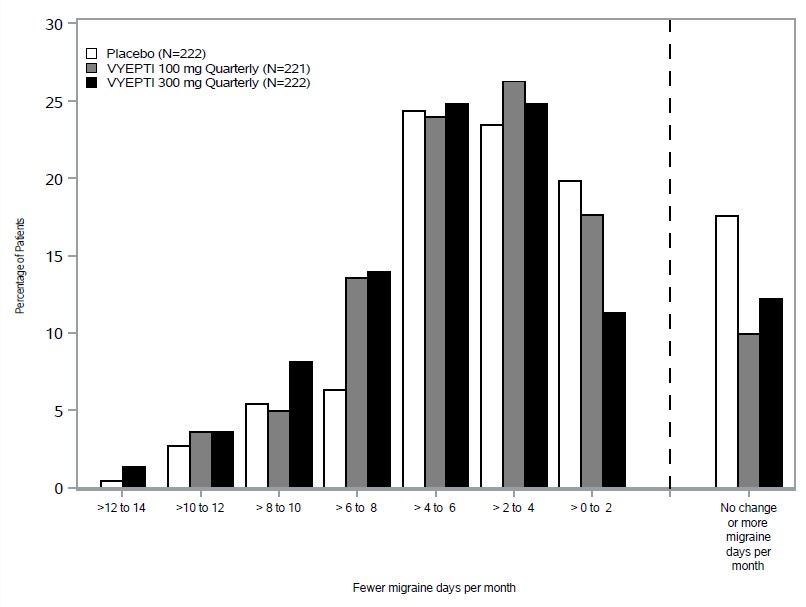
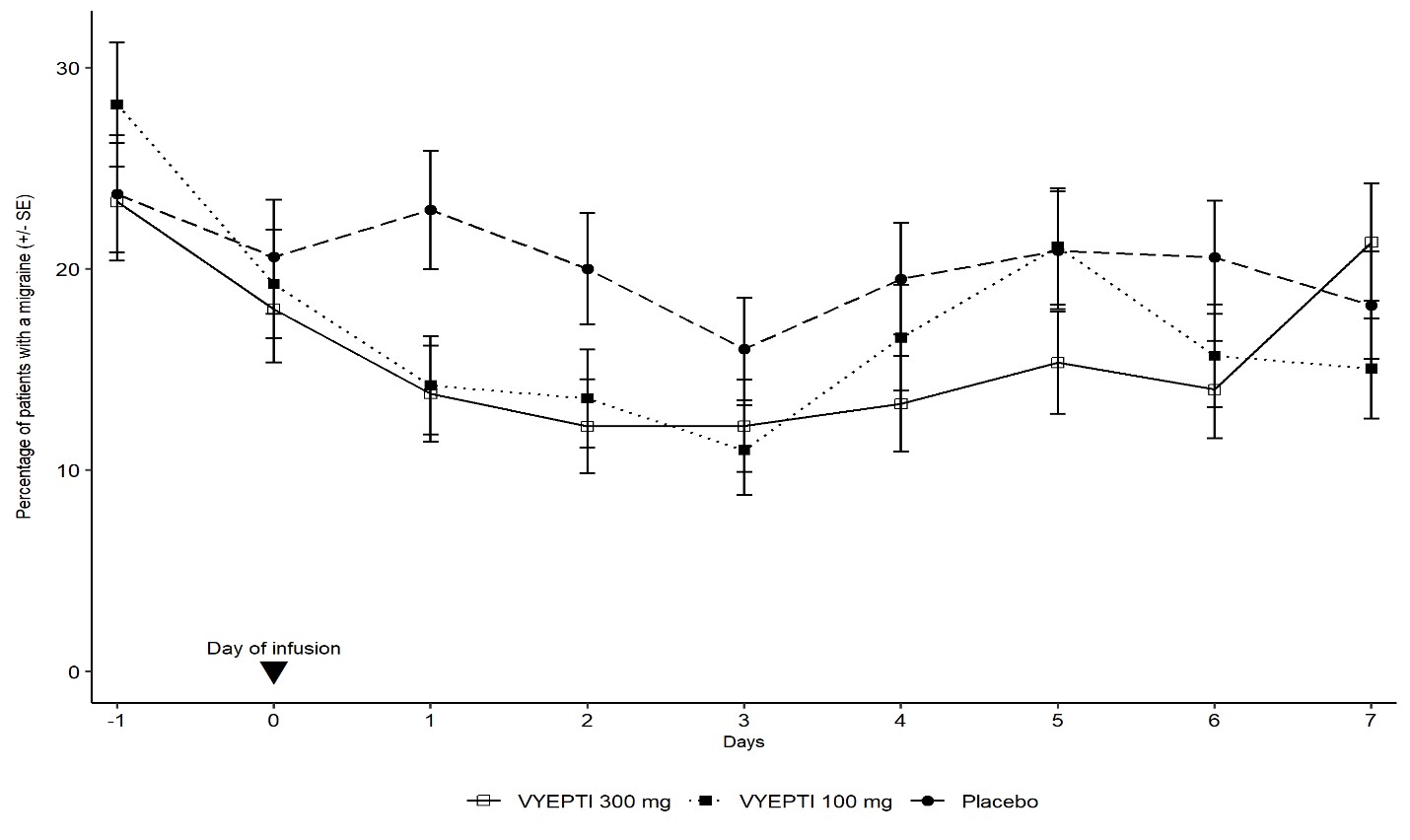
Figure 3 demonstrates that greater percentages of placebo-treated patients had migraines on most days during the first 7 days of treatment compared to VYEPTI-treated patients in Study 1.
Figure 3. Percentage of Patients with a Migraine from Day -1 (Day Prior to Infusion) to Day 7 in Study 1
Study 2: Chronic Migraine
Study 2 (NCT02974153) included adults with a history of chronic migraine (15 to 26 headache days per month, of which at least 8 were migraine days). A total of 1072 patients were randomized and received placebo (N=366), 100 mg VYEPTI (N=356), or 300 mg VYEPTI (N=350) every 3 months for 6 months. Patients were allowed to use and to continue an established stable regimen of acute migraine or headache preventive medication (except onabotulinumtoxinA). Patients with a dual diagnosis of chronic migraine and medication overuse headache attributable to acute medication overuse (triptans, ergotamine, or combination analgesics greater than 10 days per month) were included in the study population. Patients using opioids or butalbital-containing products greater than 4 days per month were not allowed.
The study excluded patients with a history of cardiovascular disease (hypertension, ischemic heart disease), neurological disease, or cerebrovascular disease.
The primary efficacy endpoint was the change from baseline in mean monthly migraine days over Months 1-3. Secondary endpoints included the percentages of patients with 50% or greater and 75% or greater reductions from baseline in monthly migraine days over Months 1-3.
Patients had a median age of 41 years (range: 18 to 65 years), 88% were female, and 91% were white. Forty-one percent of patients were taking concomitant preventive medication for migraine. The mean migraine frequency at baseline was approximately 16.1 migraine days per month and was similar across treatment groups.
VYEPTI treatment demonstrated statistically significant improvements compared to placebo for the primary efficacy endpoint, as shown in Table 3; secondary endpoints are also summarized in Table 3.
Table 3. Efficacy Endpoint Results in Study 2
|
VYEPTI 100 mg N=356 |
VYEPTI 300 mg
N=350
|
Placebo
N=366
|
|
| Monthly Migraine Days (MMD) – Months 1-3 |
|
||
| Change from baseline | -7.7 | -8.2 | -5.6 |
| Difference from placebo | -2.0 | -2.6 | |
| p-value | <0.001 | <0.001 | |
| ≥50% MMD responders –Months 1-3 | |||
| % Responders | 57.6% | 61.4% | 39.3% |
| Difference from placebo | 18.2% | 22.1% | |
| p-value | <0.001 |
<0.001 |
|
| ≥75% MMD responders –Months 1-3 | |||
| % Responders | 26.7% | 33.1% | 15.0% |
| Difference from placebo | 11.7% | 18.1% | |
| p-value | <0.001 | <0.001 | |
Figure 4 shows the mean change from baseline in average monthly migraine days for Study 2. Patients treated with VYEPTI at both doses had greater mean decreases from baseline in mean monthly migraine days over Month 1-3 compared to placebo-treated patients.
Figure 4. Change from Baseline in Monthly Migraine Days in Study 2
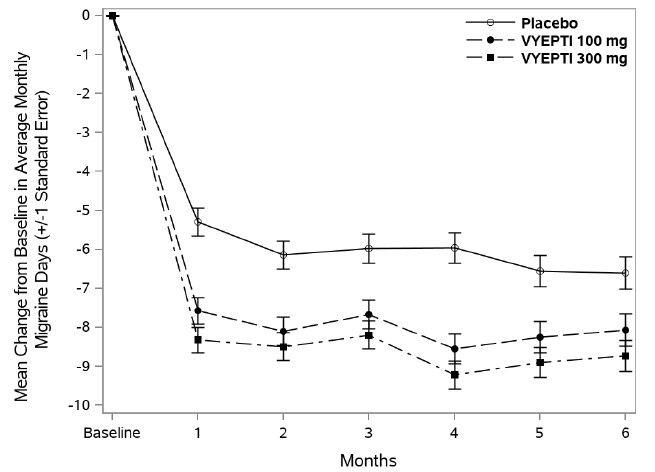
Figure 5 shows the distribution of change from baseline in mean monthly migraine days through Month 3 by treatment group in 3-day increments.
Figure 5. Distribution of Change from Baseline in Mean Monthly Migraine Days over Months 1-3 by Treatment Group in Study 2
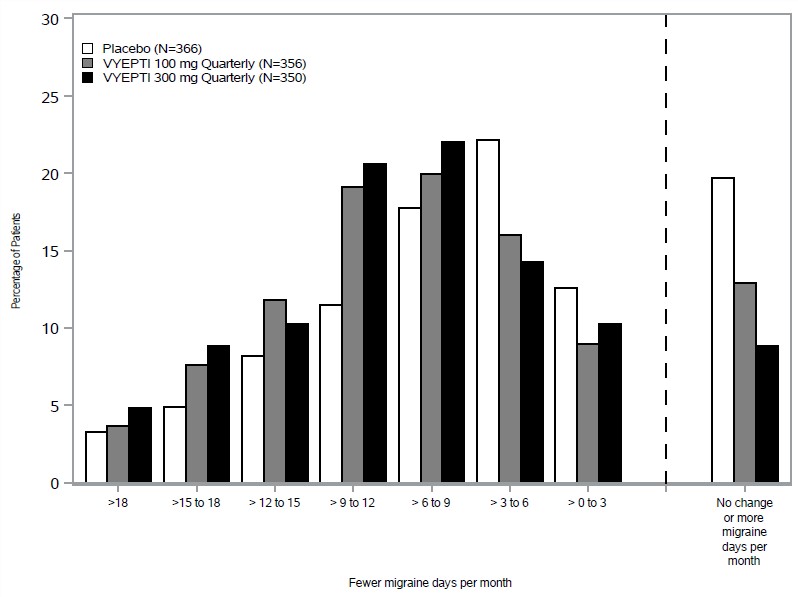
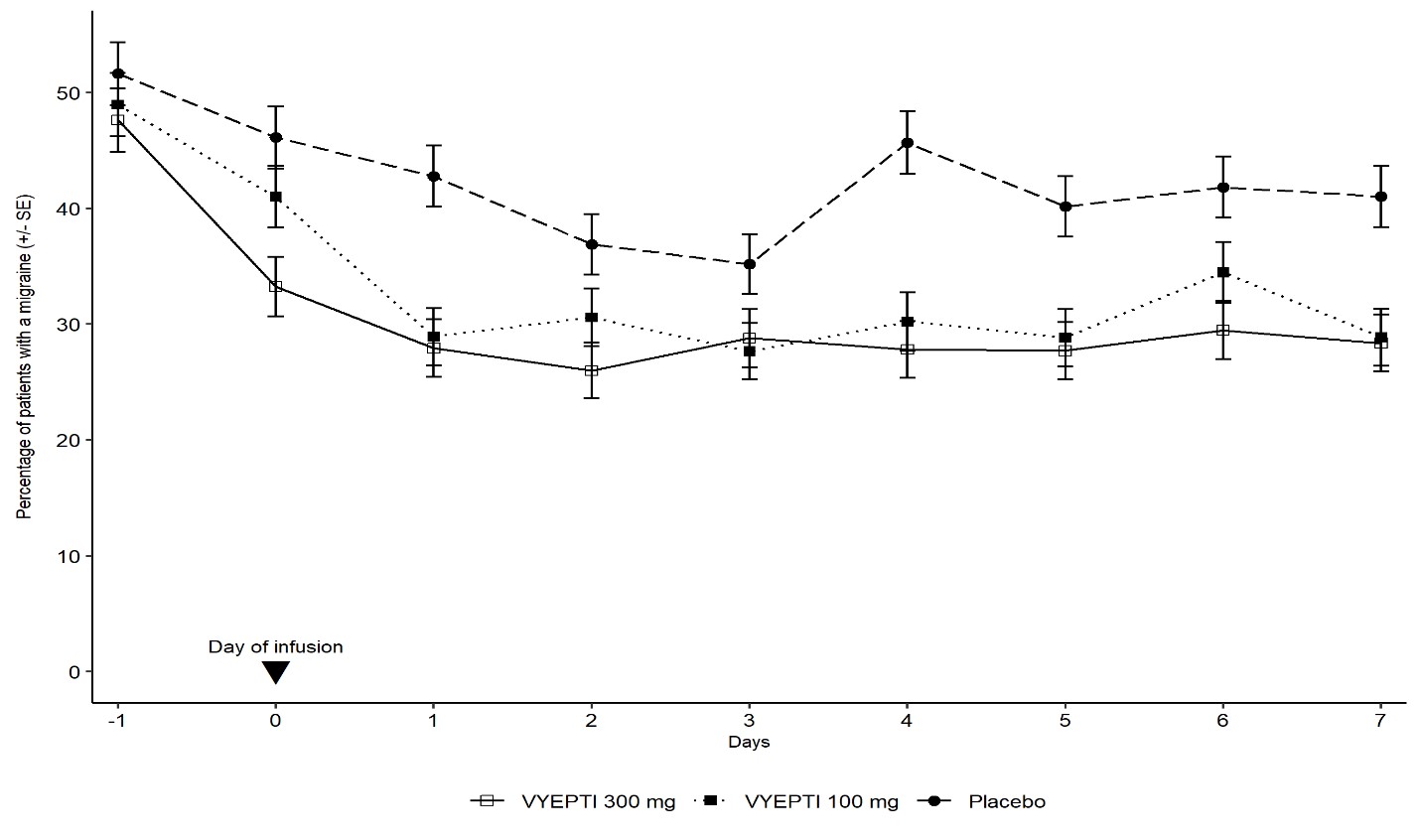
Figure 6 demonstrates that greater percentages of placebo-treated patients had migraines on individual days during the first 7 days of treatment compared to VYEPTI-treated patients in Study 2.
Figure 6. Percentage of Patients with a Migraine from Day -1 (Day Prior to Infusion) to Day 7 in Study 2
16.1 How Supplied
VYEPTI (eptinezumab-jjmr) injection is a clear to slightly opalescent, colorless to brownish-yellow solution supplied as:
Carton containing one 100 mg/mL single-dose vial - NDC 67386-130-51.
16.2 Storage and Handling
Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light until time of use. Do not freeze or shake.
The vial stopper is not made with natural rubber latex.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Hypersensitivity Reactions
Inform patients about the signs and symptoms of hypersensitivity reactions and that these reactions can occur with VYEPTI. Advise patients to contact their healthcare provider immediately if signs or symptoms of hypersensitivity reactions occur [see Warnings and Precautions (5.1)].
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant during treatment or plan to become pregnant [see Use in Specific Populations (8.1)].
Lactation
Inform patients to notify their healthcare provider if they are breastfeeding or plan to breastfeed [see Use in Specific Populations (8.2)].
Manufactured by:
Lundbeck Seattle BioPharmaceuticals, Inc.
11804 North Creek Parkway South
Bothell, WA 98011 USA
U.S. License No. 2097

Vyepti is a registered trademark of Lundbeck Seattle BioPharmaceuticals, Inc.
PATIENT PACKAGE INSERT
|
PATIENT INFORMATION VYEPTI®(vye ep' tee) (eptinezumab-jjmr) injection, for intravenous use |
|
What is VYEPTI? VYEPTI is a prescription medicine used for the preventive treatment of migraine in adults. It is not known if VYEPTI is safe and effective in children. |
|
Do not receive VYEPTI if you are allergic to eptinezumab-jjmr or any of the ingredients in VYEPTI. See the end of this Patient Information leaflet for a complete list of ingredients in VYEPTI. |
|
Before you receive VYEPTI, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. |
|
How will I receive VYEPTI?
If you have questions about your infusion schedule, ask your healthcare provider. |
|
What are the possible side effects of VYEPTI? VYEPTI may cause serious side effects, including:
The most common side effects of VYEPTI include:
These are not all of the possible side effects of VYEPTI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
General information about the safe and effective use of VYEPTI. Medicines are sometimes prescribed for purposes other than those listed in the Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about VYEPTI that is written for health professionals. |
|
What are the ingredients in VYEPTI? Active ingredient: eptinezumab-jjmr Inactive ingredients: L-histidine, L-histidine hydrochloride monohydrate, polysorbate 80, sorbitol, and Water for Injection. The vial stopper is not made with natural rubber latex.
Manufactured by: Lundbeck Seattle BioPharmaceuticals, Inc., 11804 North Creek Parkway South, Bothell, WA 98011 US License Number: 2097 |

|
| Vyepti is a registered trademark of Lundbeck Seattle BioPharmaceuticals, Inc. For more information, call 1-833-4-VYEPTI (833-489-3784) or go to www.Vyepti.com |
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 9/2021