ACUVAIL
1 INDICATIONS AND USAGE
ACUVAIL ® ophthalmic solution is indicated for the treatment of pain and inflammation following cataract surgery.
2 DOSAGE AND ADMINISTRATION
One drop of ACUVAIL should be applied by the patient to the affected eye twice daily beginning 1 day prior to cataract surgery, and continued through the first 2 weeks of the postoperative period. (2.1)
2.1 Recommended Dosing
Patient D osing
One drop of ACUVAIL should be applied to the affected eye twice daily beginning 1 day prior to cataract surgery, continued on the day of surgery, and through the first 2 weeks of the postoperative period.
2.2 Use W ith Other Topical Ophthalmic Medications
ACUVAIL ophthalmic solution may be administered in conjunction with other topical ophthalmic medications such as alpha-agonists, beta-blockers, carbonic anhydrase inhibitors, cycloplegics, and mydriatics. Drops should be administered at least 5 minutes apart.
3 DOSAGE FORMS AND STRENGTHS
4.5 mg/mL ketorolac tromethamine solution (0.45%) in a single-use vial.
4 CONTRAINDICATIONS
ACUVAIL solution is contraindicated in patients with previously demonstrated hypersensitivity to any of the ingredients in the formulation.
5.1 Delayed Healing
Topical nonsteroidal anti-inflammatory drugs (NSAIDs) may slow or delay healing. Topical corticosteroids are also known to slow or delay healing. Concomitant use of topical NSAIDs and topical steroids may increase the potential for healing problems.
5. 2 Cross-Sensitivity or Hypersensitivity
There is the potential for cross-sensitivity to acetylsalicylic acid, phenylacetic acid derivatives, and other NSAIDs. There have been reports of bronchospasm or exacerbation of asthma associated with the use of ketorolac tromethamine ophthalmic solution in patients who either have a known hypersensitivity to aspirin/non-steroidal anti-inflammatory drugs, or a past medical history of asthma. Therefore, caution should be used when treating individuals who have previously exhibited sensitivities to these drugs.
5. 3 Increased Bleeding Time
With some NSAIDs, there exists the potential for increased bleeding time due to interference with thrombocyte aggregation. There have been reports that ocularly applied nonsteroidal anti-inflammatory drugs may cause increased bleeding of ocular tissues (including hyphemas) in conjunction with ocular surgery.
It is recommended that ACUVAIL ophthalmic solution be used with caution in patients with known bleeding tendencies or who are receiving other medications, which may prolong bleeding time.
5. 4 Corneal E ffects
Use of topical NSAIDs may result in keratitis. In some susceptible patients, continued use of topical NSAIDs may result in epithelial breakdown, corneal thinning, corneal erosion, corneal ulceration, or corneal perforation. These events may be sight threatening. Patients with evidence of corneal epithelial breakdown should immediately discontinue use of topical NSAIDs and should be closely monitored for corneal health.
Postmarketing experience with topical NSAIDs suggests that patients with complicated ocular surgeries, corneal denervation, corneal epithelial defects, diabetes mellitus, ocular surface diseases (e.g., dry eye syndrome), rheumatoid arthritis, or repeat ocular surgeries within a short period of time may be at increased risk for corneal adverse events which may become sight threatening. Topical NSAIDs should be used with caution in these patients.
Postmarketing experience with topical NSAIDs also suggests that use more than 1 day prior to surgery or use beyond 14 days post-surgery may increase patient risk for the occurrence and severity of corneal adverse events.
6 ADVERSE REACTIONS
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to the rates in the clinical studies of another drug and may not reflect the rates observed in practice.
6.1 Clinical Studies Experience
The most common adverse reactions were reported in 1-6% of patients and included increased intraocular pressure, conjunctival hyperemia and/or hemorrhage, corneal edema, ocular pain, headache, tearing and vision blurred. Some of these reactions may be the consequence of the cataract surgical procedure.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postmarketing use of ketorolac tromethamine ophthalmic solutions in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. The reactions, which have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to topical ketorolac tromethamine ophthalmic solutions or a combination of these factors, include bronchospasm, exacerbation of asthma, corneal erosion, corneal perforation, corneal thinning and corneal melt, epithelial breakdown [see Warnings and Precautions ( 5.2 , 5.4 )] and ulcerative keratitis.
8 USE IN SPECIFIC POPULATIONS
Teratogenic Effects.
Pregnancy Category C : Ketorolac tromethamine, administered during organogenesis, was not teratogenic in rabbits and rats at oral doses of 3.6 mg/kg/day and 10 mg/kg/day, respectively. These doses are approximately 600 times and 1700 times higher respectively than the typical human topical ophthalmic daily dose of 0.35 mg (4.5 mg/mL x 0.04 mL/drop, twice daily) to an affected eye on a mg/kg basis. Additionally, when administered to rats after Day 17 of gestation at oral doses up to 1.5 mg/kg/day (approximately 300 times the typical human topical ophthalmic daily dose), ketorolac tromethamine resulted in dystocia and increased pup mortality. There are no adequate and well-controlled studies in pregnant women. ACUVAIL solution should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic E ffects : Because of the known effects of prostaglandin-inhibiting drugs on the fetal cardiovascular system (closure of the ductus arteriosus), the use of ACUVAIL solution during late pregnancy should be avoided.
8.1 Pregnancy
Teratogenic Effects.
Pregnancy Category C : Ketorolac tromethamine, administered during organogenesis, was not teratogenic in rabbits and rats at oral doses of 3.6 mg/kg/day and 10 mg/kg/day, respectively. These doses are approximately 600 times and 1700 times higher respectively than the typical human topical ophthalmic daily dose of 0.35 mg (4.5 mg/mL x 0.04 mL/drop, twice daily) to an affected eye on a mg/kg basis. Additionally, when administered to rats after Day 17 of gestation at oral doses up to 1.5 mg/kg/day (approximately 300 times the typical human topical ophthalmic daily dose), ketorolac tromethamine resulted in dystocia and increased pup mortality. There are no adequate and well-controlled studies in pregnant women. ACUVAIL solution should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic E ffects : Because of the known effects of prostaglandin-inhibiting drugs on the fetal cardiovascular system (closure of the ductus arteriosus), the use of ACUVAIL solution during late pregnancy should be avoided.
8.3 Nursing Mothers
Because many drugs are excreted in human milk, caution should be exercised when ACUVAIL is administered to a nursing woman.
8.5 Geriatric Use
No overall clinical differences in safety or effectiveness have been observed between elderly and other adult patients.
11 DESCRIPTION
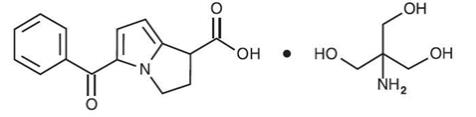
ACUVAIL (ketorolac tromethamine ophthalmic solution) 0.45% is a member of the pyrrolo-pyrrole group of nonsteroidal anti-inflammatory drugs (NSAIDs) for ophthalmic use. Its chemical name is (±)-5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid, compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1), and its molecular weight is 376.40. Its molecular formula is C19H24N2O6. Its chemical structure is:
ACUVAIL solution is supplied as a sterile isotonic aqueous 0.45% preservative-free solution, with a pH of approximately 6.8. ACUVAIL solution contains a racemic mixture of R-(+) and S-(-)- ketorolac tromethamine. Ketorolac tromethamine may exist in three crystal forms. All forms are equally soluble in water. The pKa of ketorolac is 3.5. This white to off-white crystalline substance discolors on prolonged exposure to light. The osmolality of ACUVAIL solution is approximately 285 mOsml/kg.
Each mL of ACUVAIL ophthalmic solution contains: Active: ketorolac tromethamine 0.45%. Inactives: carboxymethylcellulose sodium; sodium chloride; sodium citrate dihydrate; and purified water with sodium hydroxide and/or hydrochloric acid to adjust pH.
12 CLINICAL PHARMACOLOGY
Ketorolac tromethamine is a nonsteroidal anti-inflammatory drug which, when administered systemically, has demonstrated analgesic, anti-inflammatory, and anti-pyretic activity. The mechanism of its action is thought to be due to its ability to inhibit prostaglandin biosynthesis.
12.1 Mechanism of Action
Ketorolac tromethamine is a nonsteroidal anti-inflammatory drug which, when administered systemically, has demonstrated analgesic, anti-inflammatory, and anti-pyretic activity. The mechanism of its action is thought to be due to its ability to inhibit prostaglandin biosynthesis.
12.3 Pharmacokinetics
Two drops of 0.5% ketorolac tromethamine ophthalmic solution instilled into the eyes of patients 12 hours and 1 hour prior to cataract extraction achieved a mean ketorolac concentration of 95 ng/mL in the aqueous humor of 8 of 9 eyes tested (range 40 to 170 ng/mL).
One drop of 0.5% ketorolac tromethamine ophthalmic solution was instilled into 1 eye and 1 drop of vehicle into the other eye three times daily in 26 healthy subjects. Five (5) of 26 subjects had detectable concentrations of ketorolac in their plasma (range 11 to 23 ng/mL) at Day 10 during topical ocular treatment. The range of concentrations following three times daily dosing of 0.5% ketorolac tromethamine ophthalmic solution are approximately 4 to 8% of the steady state mean minimum plasma concentration observed following four times daily oral administration of 10 mg ketorolac in humans (290 ± 70 ng/mL).
13 NONCLINICAL TOXICOLOGY
Ketorolac tromethamine was not carcinogenic in either rats given up to 5 mg/kg/day orally for 24 months or in mice given 2 mg/kg/day orally for 18 months. These doses are approximately 900 times and 300 times higher respectively than the typical human topical ophthalmic daily dose given as twice daily to an affected eye on a mg/kg basis.
Ketorolac tromethamine was not mutagenic in vitro in the Amesassay or in forward mutation assays. Similarly, it did not result in an in vitro increase in unscheduled DNA synthesis or an in vivo increase in chromosome breakage in mice. However, ketorolac tromethamine did result in an increased incidence in chromosomal aberrations in Chinese hamster ovary cells.
Ketorolac tromethamine did not impair fertility when administered orally to male and female rats at doses up to 9 mg/kg/day and 16 mg/kg/day, respectively. These doses are respectively 1500 and 2700 times higher than the typical human topical ophthalmic daily dose.
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Ketorolac tromethamine was not carcinogenic in either rats given up to 5 mg/kg/day orally for 24 months or in mice given 2 mg/kg/day orally for 18 months. These doses are approximately 900 times and 300 times higher respectively than the typical human topical ophthalmic daily dose given as twice daily to an affected eye on a mg/kg basis.
Ketorolac tromethamine was not mutagenic in vitro in the Amesassay or in forward mutation assays. Similarly, it did not result in an in vitro increase in unscheduled DNA synthesis or an in vivo increase in chromosome breakage in mice. However, ketorolac tromethamine did result in an increased incidence in chromosomal aberrations in Chinese hamster ovary cells.
Ketorolac tromethamine did not impair fertility when administered orally to male and female rats at doses up to 9 mg/kg/day and 16 mg/kg/day, respectively. These doses are respectively 1500 and 2700 times higher than the typical human topical ophthalmic daily dose.
14 CLINICAL STUDIES
Two multicenter, randomized, double-masked, parallel group comparison studies including approximately 500 patients were conducted to evaluate the effects of ACUVAIL on anterior chamber cell and flare, and ocular pain relief following cataract extraction with posterior chamber intraocular lens (IOL) implantation. Results of these studies indicated that patients receiving ACUVAIL had a significantly higher incidence of clearing of anterior chamber inflammation 53% (167/318) versus patients receiving vehicle 26% (41/155) at day 14.
ACUVAIL was also significantly superior to vehicle in resolving ocular pain. On Day 1 post cataract surgery, 72% (233/322) of patients in the ACUVAIL group were pain free compared to 40% (62/156) of patients in the vehicle group.
Results from clinical studies indicate that ketorolac tromethamine has no significant effect upon intraocular pressure; however, changes in intraocular pressure may occur following cataract surgery.
16 HOW SUPPLIED/STORAGE AND HANDLING
ACUVAIL (ketorolac tromethamine ophthalmic solution) 0.45% is available as a sterile solution supplied in clear, LDPE, single-use vials packaged in 3 foil pouches, 10 vials per pouch:
30 Single-Use Vials 0.4 mL each: NDC 0023-3507-31
Storage : Store at 15o-30º C (59o-86º F). Store the vials in the pouch, protected from light. Fold pouch ends closed.
17 PATIENT COUNSELING INFORMATION
Patients should be informed of the possibility that slow or delayed healing may occur while using nonsteroidal anti-inflammatory drugs (NSAIDs).
17.1 Slow or Delayed Healing
Patients should be informed of the possibility that slow or delayed healing may occur while using nonsteroidal anti-inflammatory drugs (NSAIDs).
17.2 Avoiding Contamination of the Product
Patients should be instructed that the solution from one individual single-use vial is to be used immediately after opening for administration to the affected eye. The remaining vial contents should be discarded.
The use of the same single-use vial of topical eye drops for both eyes following bilateral ocular surgery is not recommended. In these circumstances, advise patients to use one vial for each eye immediately after opening and discard the remaining contents after use.
Patients should be instructed to avoid allowing the tip of the vial to contact the eye or surrounding structures because this could cause the tip to become contaminated by common bacteria known to cause ocular infections or cause injury to the eye. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions.
Store the vials in the pouch, protected from light. Fold pouch ends closed.
17. 3 Contact Lens Wear
Patients should be advised that ACUVAIL solution should not be administered while wearing contact lenses.
17.4 Intercurrent Ocular Conditions
Patients should be advised that if they develop an intercurrent ocular condition (e.g., trauma or infection) or have ocular surgery, they should immediately seek their physician’s advice concerning the continued use of ACUVAIL ®.
17.5 Concomitant Topical Ocular Therapy
Patients should be advised that if more than one topical ophthalmic medication is being used, the medicines should be administered at least 5 minutes apart.
Distributed by: Allergan USA, Inc.
Madison, NJ 07940
© 2019 Allergan. All rights reserved.
All trademarks are the property of their respective owners.
Patented. See: www.allergan.com/patents
v2.0USPI3507