Therapeutic drug levels
Labs - DRUG Levels (Alphabetical order)
Therapeutic Range: 5-20 mcg/mL
Comments:
Potentially toxic / Critical value:
>150 mcg/mL - Measured 4 hours after the dose.
The ceiling analgesic effect is obtained with a dose of 1 gram (adult).
Rumack-Matthew nomogram for single acute acetaminophen poisoning:
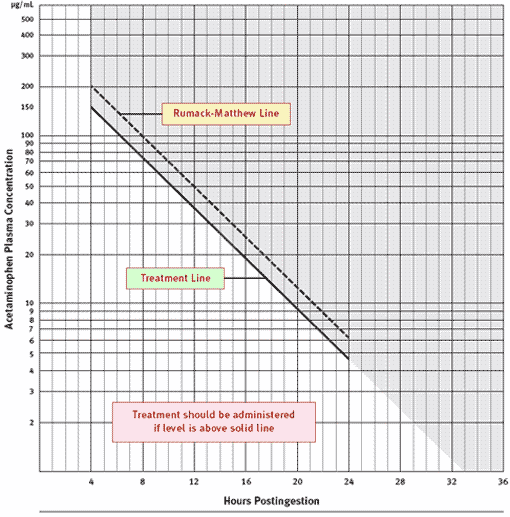
Recommended reading / References:
Acetadote® (acetylcysteine) Injection. Package insert. Cumberland Pharmaceuticals Inc. Nashville, TN 37203. Feb 2006.
Rumack BH, Matthew M. Acetaminophen poisoning and toxicity. Pediatrics 1975; 55:871–876
Rumack BH, Peterson RC, Koch GG, Amara IA. Acetaminophen overdose: 662 cases with evaluation of oral acetylcysteine treatment. Arch Intern Med. 1981;141:380-385.
Amikacin: 
Therapeutic levels:
Peak: ~20-30 mcg/ml [Life-threatening infections: 25 to 40 mcg/mL]
Trough: 4-8 mcg/ml
Potentially toxic:
peak: >30.0 mcg/mL,
trough: >8.0 mcg/mL.
Toxic concentration:
Peak: >40 mcg/mL;
Trough: >10 mcg/mL
Half-life:
Children: 1.6 to 2.5 hours
Adults with normal renal function: ~2-3 hours
ESRD: 17 to 150 hours
Monitoring:
Drawing levels:
Draw peak 30 minutes after a 30 infusion or 60 minutes after an IM injection.
Draw trough within 30 minutes prior to next dose or at least one half-life after the peak.
Extended-interval (once-daily) dosing: Draw a random amikacin level ~6 to 14 hours after the start of the amikacin infusion. Use an approved nomogram for additional guidance.
Other: serum creatinine, BUN, weight, temperature, I&O’s,
Evaluation:
Initial evaluation Is the drug appropriate?
Is the dose appropriate?
Specific patient parameters
(a) Age, sex, height, weight, IBW.
(b) Allergies
(c) Current antibiotic therapy
(d) Infectious diagnosis
(e) Renal and hepatic function
(f ) Concurrent disease states/diagnoses that may impact therapy
(eg. cachexia, diabetes mellitus, bilateral AKA, etc.)
(g) Clinical signs and symptoms (Temp, RR, HR etc)
(h) Laboratory tests: gram stains, culture and sensitivity results, CBC & diff,
Scr, BUN, WBC, I/O (past 24 hours)
(i ) Determination of optimal blood levels based on diagnosis.
Follow-up Evaluation
Is the drug working?
Is the patient experiencing any adverse effects from the drug?
Treatment failure:
Temperature or WBC increasing; symptoms increasing.
Note: changes in renal function may not reflect nephrotoxicity.
Other causes of acute renal failure occurring
in hospitalized patients, include:
- Severe or prolonged hypotension (decreased renal perfusion)
- Surgery
- Other nephrotoxic drugs: amphotericin, cisplatin, etc.
- Acute cardiovascular dysfunction
Is the patient responding to treatment?
Look for reversal of initial signs and symptoms.
-Is the patient's temperature decreasing?
Culture results negative?
-Heart rate and respiratory rate returning to normal?
-Is the white blood cell count decreasing?
-Normalization of blood gases?
Is the current regimen appropriate?
Is the patients renal function stable?
Are current serum levels (peak and trough) appropriate?
Is the drug still required?
Overview of adverse effects:
Aminoglycosides:
Major: Vestibular toxicity; auditory toxicity;
renal toxicity (reversible); neuromuscular toxicity (post-synaptic
curare-like action).
Minor: skin rash; drug induced fever.
What to look for: Changes in urine output; BUN,
creatinine, ototoxicity (hearing tests).]
Monitoring for toxicity:
1. Auditory and neuromuscular toxicity are not evaluated in most patients.
If prolonged courses of aminoglycosides are anticipated, baseline and
periodic assessment of hearing with audiometry are recommended.
Neuromuscular toxicity is most likely in patients with preexisting
neuromuscular disease or patients with hypocalcemia.
2. Serum creatinine and BUN determinations 2-3 times/week should monitor
renal toxicity. More frequent determinations are advised for patients with
changing renal function. Creatinine clearance, I/O's, urinalysis, when
available will help to identify patients with possible nephrotoxicity.
3. Risk factors for Nephrotoxicity: Age, renal insufficiency, elevated trough
concentrations, total daily dose, cumulative dose, concurrent nephrotoxic
drugs, prior aminoglycoside exposure, duration of treatment.
4. Toxicity usually takes 5-7 days to develop. Mechanism: damage occurs
to the cells lining the proximal tubules.
5. The process of nephrotoxicity (uptake by cells) is saturable and the
number of insults determines toxicity. It is imperative to minimize the
number of insults and allow the tubular cells a relatively drug free
period in which to regenerate cells.
Amitriptyline 
Therapeutic levels:
Amitriptyline plus nortriptyline 80-250 ng/mL
Half-life: 17-40 hours
Steady state: 3-8 days.
Toxic level/ Increased risk of side effects:
amitriptyline plus nortriptyline levels >300 ng/mL
Drawing levels:
Draw trough immediately prior to next dose
Carbamazepine 
Therapeutic levels:
4 to 12 mcg/ml [SI: 17 to 51 micromole/L]
Note: 4 to 8 mcg/mL if patient is receiving other anticonvulsants.
Half-life:
8 - 60 hours in adults
Epoxide metabolite: 25 to 43 hours (active metabolite)
Time to Steady state: variable - autoinduction is usually complete in 2 to 5 weeks after a fixed regimen.
Autoinduction: liver enzymes are induced --> increased metabolism and shorter half-life.
cytochrome P450 3A4
Toxic level: >15 mcg/ml
Drawing levels:
Draw trough immediately prior to next dose.
Monitoring:
CBC with platelet count and differential
LFT's
Renal fcn
BUN / Scr
Ophthalmic exam (periodic).
Desipramine 
Therapeutic levels:
150 to 300 ng/ml
Toxic level: >500 ng/ml
Half-life: 15-24 hours
Onset of action: 4 to 8 weeks to determine response
Drawing levels:
obtain trough just prior to next dose.
Monitoring:
CBC
blood glucose
vitals
mental status
BMI (weight)
ECG (Elderly, cardiac dx)
Digoxin 
Therapeutic levels:
0.8 to 2.0 ng/ml
Heart failure: 0.5 to 0.9 ng/mL
Half-life:
Adults: 36 to 48 hours [anephric: 3.5 to 5 days]
Children: 18 to 36 hours
Time to Steady state: 5 - 10 days (possibly longer) - ESRD: 15 to 20 days
Toxic level: >2 ng/ml
Drawing levels:
-Determine serum digoxin levels at least 6 to 8 hours after the last regardless of the route of administration in order to allow sufficient time for drug distribution.
-Optimal timing: 12 to 24 hours after a dose or initial loading dose.
-If a loading dose is omitted, a digoxin level should be obtained after 3 to 5 days of therapy.
-A digoxin serumn level should be obtained within 5 to 7 days after any dosage changes - continue assessment 7 - 14 days after any change in the maintenance dose.
-Follow-up levels should be obtained for any of the following: fluctuating renal function; suspected toxicity; concomitant conditions change; interacting drugs added or removed; suspected changes in electrolytes (Mg++, K+, or Ca++).
Disopyramide 
Therapeutic levels:
Atrial arrhythmias: 2.8-3.2 mcg/mL
Ventricular arrhythmias 3.3-7.5 mcg/mL
Half-life: 4 - 10 hours
[prolonged: heart failure and renal/hepatic failure]
Toxic level: >7 mcg/ml
Drawing levels:
Trough: immediately prior to next dose.
Ethosuximide 
Therapeutic levels:
40 to 100 mcg/mL
Half-life:
Children: 30 hours
Adults: 50 to 60 hours
Time to Steady state: 7-10 days.
Toxic level: 100-150 mcg/ml
Drawing levels:
Obtaining levels: peak: 2-4 hours after the dose. Trough: immediately prior to next dose.
Gentamicin 
Administration:
IM: deep IM
IV: 30-120 minutes
Therapeutic levels:
Conventional dosing:
Peak: 4-10 mcg/ml [8 mcg/mL or less if not life-threatening]
Trough: 0.5 to 2 mcg/ml [0.5 to 1 mcg/mL if not life-threatening]
Half-life: (~ 2 - 3 hours).
ESRD: 17 to 65 hrs or longer
Children: 1 - 3 hours
Time to Steady state: variable
Toxic level:
Drawing levels:
Draw peak 30 minutes after a 30 infusion or 60 minutes after an IM injection. Draw trough just prior to next dose or at least one half-life after the peak.
Evaluation:
Initial evaluation Is the drug appropriate?
Is the dose appropriate?
Specific patient parameters
(a) Age, sex, height, weight, IBW.
(b) Allergies
(c) Current antibiotic therapy
(d) Infectious diagnosis
(e) Renal and hepatic function
(f ) Concurrent disease states/diagnoses that may impact therapy
(eg. cachexia, diabetes mellitus, bilateral AKA, etc.)
(g) Clinical signs and symptoms (Temp, RR, HR etc)
(h) Laboratory tests: gram stains, culture and sensitivity results, CBC & diff,
Scr, BUN, WBC, I/O (past 24 hours)
(i ) Determination of optimal blood levels based on diagnosis.
Follow-up Evaluation
Is the drug working?
Is the patient experiencing any adverse effects from the drug?
Treatment failure:
Temperature or WBC increasing; symptoms increasing.
Note: changes in renal function may not reflect nephrotoxicity.
Other causes of acute renal failure occurring
in hospitalized patients, include:
- Severe or prolonged hypotension (decreased renal perfusion)
- Surgery
- Other nephrotoxic drugs: amphotericin, cisplatin, etc.
- Acute cardiovascular dysfunction
Is the patient responding to treatment?
Look for reversal of initial signs and symptoms.
-Is the patient's temperature decreasing?
Culture results negative?
-Heart rate and respiratory rate returning to normal?
-Is the white blood cell count decreasing?
-Normalization of blood gases?
Is the current regimen appropriate?
Is the patients renal function stable?
Are current serum levels (peak and trough) appropriate?
Is the drug still required?
Overview of adverse effects:
Aminoglycosides:
Major: Vestibular toxicity; auditory toxicity;
renal toxicity (reversible); neuromuscular toxicity (post-synaptic
curare-like action).
Minor: skin rash; drug induced fever.
What to look for: Changes in urine output; BUN,
creatinine, ototoxicity (hearing tests).]
Monitoring for toxicity:
1. Auditory and neuromuscular toxicity are not evaluated in most patients.
If prolonged courses of aminoglycosides are anticipated, baseline and
periodic assessment of hearing with audiometry are recommended.
Neuromuscular toxicity is most likely in patients with preexisting
neuromuscular disease or patients with hypocalcemia.
2. Serum creatinine and BUN determinations 2-3 times/week should monitor
renal toxicity. More frequent determinations are advised for patients with
changing renal function. Creatinine clearance, I/O's, urinalysis, when
available will help to identify patients with possible nephrotoxicity.
3. Risk factors for Nephrotoxicity: Age, renal insufficiency, elevated trough
concentrations, total daily dose, cumulative dose, concurrent nephrotoxic
drugs, prior aminoglycoside exposure, duration of treatment.
4. Toxicity usually takes 5-7 days to develop. Mechanism: damage occurs
to the cells lining the proximal tubules.
5. The process of nephrotoxicity (uptake by cells) is saturable and the
number of insults determines toxicity. It is imperative to minimize the
number of insults and allow the tubular cells a relatively drug free
period in which to regenerate cells.
Imipramine 
Therapeutic levels:
150 to 250 ng/ml
Half-life: 9-24 hours
Time to Steady state: 2-5 days
Toxic level: >250 ng/ml
Lidocaine 
Therapeutic levels:
1.5 to 5.0 mcg/ml
Half-life:
Adults: 1.5 to 2 hours
Time to Steady state:
6-12 hours
Potentially Toxic level:
>6 mcg/ml
Lithium 
Therapeutic levels:
0.5 to 1.2 mEq/L
[Acute mania: 0.5 to 1.2 mEq/L]
[Maintenance: 0.6 to 1 mEq/L]
Half-life:
Adults: 18-36 hours.
Potentially Toxic level:
>1.5 mEq/L
Drawing levels:
-Just prior to next dose or at least 8-12 hours after the last dose.
-Obtain levels two times per week until levels and patient's clinical status are stable, then monitor levels no less than every 6 months.
Nortriptyline 
Therapeutic levels:
50 to 150 ng/ml
Half-life: 15-90 hours
Time to Steady state: 4-20 days.
Drawing levels:
Just prior to next dose
Phenobarbital 
Therapeutic levels:
10 to 40 mcg/ml
Half-life:
Adults: 53 to 118 hours
Infants and Children: 60 to 180 hours
Time to Steady state:
15-25 days
Potentially Toxic level:
>40 mcg/ml
Drawing levels:
Peak: 4-12 hours after dose.
Trough: immediately prior to next dose.
Phenytoin 
Therapeutic levels:
Total: 10 to 20 mg/L
Free: 1 to 2.5 mg/L.
Half-life:
7 - 42 hours (average: 24 hours)
Time to Steady state:
7-10 days.
Potentially Toxic level:
>20-30 mcg/ml [ Toxic: >30 mg/L ]
Drawing levels:
IV: 2-4 hours after loading dose.
Oral (peak): 3-9 hours after dose (not critical).
Trough: immediately prior to next dose.
Therapy initiation: Draw within 5 to 8 days of therapy initiation and adjust as needed.
Long-term - stable patients: monitor levels every 3 to 12 months.
Primidone 
Therapeutic levels:
5 to 12 mcg/ml
Half-life:
5 to16 hours (varies with age)
Converted into two active metabolites: phenobarbital and phenylethylmalonamide (PEMA).
PEMA: 16 to 50 hours
Phenobarbital: 50 to 150 hours
Potentially Toxic level:
>12 mcg/ml
[Toxic: >15 mcg/mL]
Procainamide 
Therapeutic levels:
4 to 10 mcg/ml
NAPA: 15 - 25 mcg/ml
Combined: 10 to 30 mcg/mL
Half-life:
2.5 - 5 hours
Time to Steady state:
12 to 18 hours
Potentially Toxic level:
>10 to 12 mcg/mL
Drawing levels:
IV: 6 to 12 hours after IV infusion has started
Quinidine 
Therapeutic levels:
2 to 5 mcg/ml
Half-life:
4-11 hours (prolonged: elderly, CHF, cirrhosis.)
Adults: 6 to 8 hours
Children: 2.5 to 6.7 hours
Potentially Toxic level:
>6 mcg/ml
Drawing levels:
Draw trough level: immediately prior to next dose
Salicylate 
Therapeutic levels:
5-25 mg/dL
Analgesic: ~10 mg/dL
Anti-inflammatory: 15 -30 mg/dL
Potentially Toxic levels:
Mild toxicity: ~ 40 mg/dL
Severe toxicity: >80 mg/dL
Drawing levels:
4-6 hours after dose or just prior to next dose
Theopylline 
Therapeutic levels:
Asthma:
Children: 5-10 mcg/mL
Adults: 5-15 mcg/mL
Half-life:
3 -10 hours
Time to Steady state:
36 hours (average).
Potentially Toxic level:
>20 mcg/ml
Drawing levels: (after steady state achieved):
Oral solution or immediate-release tablet:
1-2 hours after administration.
Extended-release tablet:
4-12 hours after administration.
Recheck levels 1-2 days after any dosage change (adults).
Additional Info:
THEOPHYLLINE IN DEXTROSE (theophylline anhydrous) injection, solution
[Baxter Healthcare Corporation] Package Insert (accessed 9/16/2014):
In patients who have received no theophylline in the previous 24 hours, a serum concentration should be measured 30 minutes after completion of the intravenous loading dose to determine whether the serum concentration is <10 mcg/mL indicating the need for an additional loading dose or >20 mcg/mL indicating the need to delay starting the constant IV infusion. Once the infusion is begun, a second measurement should be obtained after one expected half-life (e.g., approximately 4 hours in children age 1 to 9 years and 8 hours in nonsmoking adults; See package insert for the expected half-life in additional patient populations). The second measurement should be compared to the first to determine the direction in which the serum concentration has changed. The infusion rate can then be adjusted before steady-state is reached in an attempt to prevent an excessive or sub-therapeutic theophylline concentration from being achieved. If a patient has received theophylline in the previous 24 hours, the serum concentration should be measured before administering an intravenous loading dose to make sure that it is safe to do so. If a loading dose is not indicated (i.e., the serum theophylline concentration is >/=10 mcg/mL), a second measurement should be obtained as above at the appropriate time after starting the intravenous infusion. If, on the other hand, a loading dose is indicated, a second blood sample should be obtained after the loading dose and a third sample should be obtained one expected half-life after starting the constant infusion to determine the direction in which the serum concentration has changed.
Once the above procedures related to initiation of intravenous theophylline infusion have been completed, subsequent serum samples for determination of theophylline concentration should be obtained at 24-hour intervals for the duration of the infusion. The theophylline infusion rate should be increased or decreased as appropriate based on the serum theophylline levels. When signs or symptoms of theophylline toxicity are present, the intravenous infusion should be stopped and a serum sample for theophylline concentration should be obtained as soon as possible, analyzed immediately, and the result reported to the clinician without delay. In patients in whom decreased serum protein binding is suspected (e.g., cirrhosis, women during the third trimester of pregnancy), the concentration of unbound theophylline should be measured and the dosage adjusted to achieve an unbound concentration of 6-12 mcg/mL.
Tobramycin 
Administration:
IV: 20-60 minutes
Therapeutic levels:
Conventional dosing:
Peak: 4-10 mcg/ml
[8 to 10 mcg/mL reserved for life-threatening infections]
Trough: 0.5 to 2 mcg/ml
Serious infections: 0.5 to 1 mcg/mL
Life-threatening infections: 1 to 2 mcg/mL
Half-life:
Adults: (average 2-3 hours). ESRD: up to 70 hours
Drawing levels:
Draw peak 30 minutes after a 30 infusion or 60 minutes after an IM injection.
Draw trough just prior to next dose or at least one half-life after the peak.
Follow-up Evaluation
Is the drug working?
Is the patient experiencing any adverse effects from the drug?
Treatment failure:
Temperature or WBC increasing; symptoms increasing.
Note: changes in renal function may not reflect nephrotoxicity.
Other causes of acute renal failure occurring
in hospitalized patients, include:
- Severe or prolonged hypotension (decreased renal perfusion)
- Surgery
- Other nephrotoxic drugs: amphotericin, cisplatin, etc.
- Acute cardiovascular dysfunction
Is the patient responding to treatment?
Look for reversal of initial signs and symptoms.
-Is the patient's temperature decreasing?
Culture results negative?
-Heart rate and respiratory rate returning to normal?
-Is the white blood cell count decreasing?
-Normalization of blood gases?
Is the current regimen appropriate?
Is the patients renal function stable?
Are current serum levels (peak and trough) appropriate?
Is the drug still required?
Overview of adverse effects:
Aminoglycosides:
Major: Vestibular toxicity; auditory toxicity;
renal toxicity (reversible); neuromuscular toxicity (post-synaptic
curare-like action).
Minor: skin rash; drug induced fever.
What to look for: Changes in urine output; BUN,
creatinine, ototoxicity (hearing tests).]
Monitoring for toxicity:
1. Auditory and neuromuscular toxicity are not evaluated in most patients.
If prolonged courses of aminoglycosides are anticipated, baseline and
periodic assessment of hearing with audiometry are recommended.
Neuromuscular toxicity is most likely in patients with preexisting
neuromuscular disease or patients with hypocalcemia.
2. Serum creatinine and BUN determinations 2-3 times/week should monitor
renal toxicity. More frequent determinations are advised for patients with
changing renal function. Creatinine clearance, I/O's, urinalysis, when
available will help to identify patients with possible nephrotoxicity.
3. Risk factors for Nephrotoxicity: Age, renal insufficiency, elevated trough
concentrations, total daily dose, cumulative dose, concurrent nephrotoxic
drugs, prior aminoglycoside exposure, duration of treatment.
4. Toxicity usually takes 5-7 days to develop. Mechanism: damage occurs
to the cells lining the proximal tubules.
5. The process of nephrotoxicity (uptake by cells) is saturable and the
number of insults determines toxicity. It is imperative to minimize the
number of insults and allow the tubular cells a relatively drug free
period in which to regenerate cells.
Valproic Acid 
Therapeutic levels:
Epilepsy: 50 to 100 mcg/mL
Mania: 50-125 mcg/mL
Half-life:
Adults: 9 to 19 hours
Children: 3.5 to 20 hours
Time to Steady state:
2-4 days
Potentially Toxic level:
>100 - 150 mcg/mL (highly variable)
Drawing levels:
-Within 2-4 days of initiation or dose adjustment.
-Trough concentrations should be drawn just before the next dose.
Vancomycin 
Therapeutic levels:
Peak: 20-40 mcg/ml
Trough: >10-20 mcg/ml
(complicated infections: 15 to 20 mcg/mL).
Half-life:
Adults: 4 to 6 hours. ESRD: significantly longer.
Time to Steady state:
Variable
Toxic level:
Peak: >80 mcg/mL
Drawing levels:
Draw peak at least 1 hour after a 60-minute infusion. Draw trough immediately prior to the next dose.
-Steady state monitoring: Occurs after the third dose [obtain before 4th dose].
Evaluation:
Initial evaluation Is the drug appropriate?
Is the dose appropriate?
Specific patient parameters
(a) Age, sex, height, weight, IBW.
(b) Allergies
(c) Current antibiotic therapy
(d) Infectious diagnosis
(e) Renal and hepatic function
(f ) Concurrent disease states/diagnoses that may impact therapy
(eg. cachexia, diabetes mellitus, bilateral AKA, etc.)
(g) Clinical signs and symptoms (Temp, RR, HR etc)
(h) Laboratory tests: gram stains, culture and sensitivity results, CBC & diff,
Scr, BUN, WBC, I/O (past 24 hours)
(i ) Determination of optimal blood levels based on diagnosis.
Follow-up Evaluation
Is the drug working?
Is the patient experiencing any adverse effects from the drug?
Treatment failure:
Temperature or WBC increasing; symptoms increasing.
Note: changes in renal function may not reflect nephrotoxicity.
Other causes of acute renal failure occurring
in hospitalized patients, include:
- Severe or prolonged hypotension (decreased renal perfusion)
- Surgery
- Other nephrotoxic drugs: amphotericin, cisplatin, etc.
- Acute cardiovascular dysfunction
Is the patient responding to treatment?
Look for reversal of initial signs and symptoms.
-Is the patient's temperature decreasing?
Culture results negative?
-Heart rate and respiratory rate returning to normal?
-Is the white blood cell count decreasing?
-Normalization of blood gases?
Is the current regimen appropriate?
Is the patients renal function stable?
Are current serum levels (peak and trough) appropriate?
Is the drug still required?
Overview of adverse effects:
Red man syndrome (histamine-like reaction characterized by flushing,
tingling, pruritis, tachycardia, and a red rash on the face,neck,upper
torso, back and arms.
Management: slow rate of infusion, dilute drug in sufficient diluent,
anti-histamine prophylaxis); Nephrotoxicity (reversible)-incidence
(<5%); ototoxicity (may be irreversible); neutropenia; thrombophlebitis;
skin rash.
Monitoring for toxicity:
1. Auditory and neuromuscular toxicity are not evaluated in most patients.
If prolonged courses of aminoglycosides are anticipated, baseline and
periodic assessment of hearing with audiometry are recommended.
Neuromuscular toxicity is most likely in patients with preexisting
neuromuscular disease or patients with hypocalcemia.
2. Serum creatinine and BUN determinations 2-3 times/week should monitor
renal toxicity. More frequent determinations are advised for patients with
changing renal function. Creatinine clearance, I/O's, urinalysis, when
available will help to identify patients with possible nephrotoxicity.
3. Risk factors for Nephrotoxicity: Age, renal insufficiency, elevated trough
concentrations, total daily dose, cumulative dose, concurrent nephrotoxic
drugs, prior aminoglycoside exposure, duration of treatment.
4. Toxicity usually takes 5-7 days to develop. Mechanism: damage occurs
to the cells lining the proximal tubules.
5. The process of nephrotoxicity (uptake by cells) is saturable and the
number of insults determines toxicity. It is imperative to minimize the
number of insults and allow the tubular cells a relatively drug free
period in which to regenerate cells.
References
Basic Skills in Interpreting Laboratory Data, 5th Ed. (2013) Lee M (editor). American Society of Health-System Pharmacists, ISBN 1585283436.
Drug information handbook: a comprehensive resource for all clinicians and healthcare professionals, 24th Ed. (2015) Lexi-Comp.

