Weight Management Agents
Lorcaserin hydrochloride - belviq®
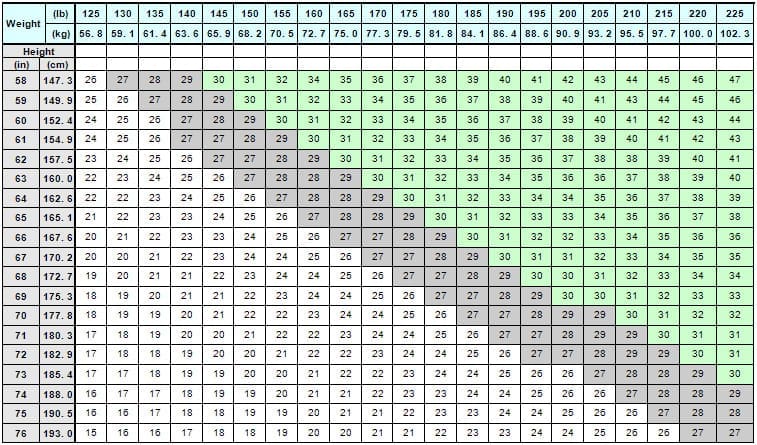
| Drug: BELVIQ (lorcaserin hydrochloride) tablets, for oral use, CIV [Drug information ] Dosing: Click (+) next to Dosage and Administration section (drug info link) Initial U.S. Approval: 2012 Mechanism of Action: INDICATIONS AND USAGE: BELVIQ is indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients with an initial body mass index (BMI) of: 30 kg/m2 or greater (obese), or [see Dosage and Administration (2)] Limitations of Use: DOSAGE AND ADMINISTRATION BELVIQ can be taken with or without food. Response to therapy should be evaluated by week 12. If a patient has not lost at least 5% of baseline body weight, discontinue BELVIQ, as it is unlikely that the patient will achieve and sustain clinically meaningful weight loss with continued treatment [see Clinical Studies (14)]. BMI is calculated by dividing weight (in kg) by height (in meters) squared. A BMI chart for height in inches and weight in pounds is provided below: Table 1. BMI Conversion Chart Drug UPDATES: BELVIQ XR ® (lorcaserin hydrochloride) extended - release tablets CIV Initial U.S. Approval: 2016 INDICATIONS AND USAGE: BELVIQ XR is a serotonin 2C receptor agonist indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adults with an initial body mass index (BMI) of: 30 kg/m2 or greater (obese) (1) or Limitations of Use: The safety and efficacy of coadministration with other products for weight loss have not been established (1) DOSAGE AND ADMINISTRATION: HOW SUPPLIED: |
Phentermine and topiramate extended-release capsules, for oral use, civ - qsymia™
| INDICATIONS AND USAGE: Qsymia is a combination of phentermine, a sympathomimetic amine anorectic, and topiramate extended-release, an antiepileptic drug, indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adults with an initial body mass index (BMI) of: 30 kg/m2 or greater (obese) or Limitations of Use: USE IN SPECIFIC POPULATIONS DOSAGE AND ADMINISTRATION: Recommended dose: Qsymia 3.75 mg/23 mg (phentermine 3.75 mg/topiramate 23 mg extended-release) daily for 14 days; then increase to 7.5 mg/46 mg daily . Discontinue or escalate dose (as described) if 3% weight loss is not achieved after 12 weeks on 7.5 mg/46 mg dose. Discontinue Qsymia if 5% weight loss is not achieved after 12 weeks on maximum daily dose of 15 mg/92 mg. Discontinue 15 mg/92 mg dose gradually (as described) to prevent possible seizure. Do not exceed 7.5 mg/46 mg dose for patients with moderate or severe renal impairment or patients with moderate hepatic impairment. DOSAGE FORMS AND STRENGTHS: CONTRAINDICATIONS: WARNINGS AND PRECAUTIONS Increase in Heart Rate: Monitor heart rate in all patients, especially those with cardiac or cerebrovascular disease . Suicidal Behavior and Ideation: Monitor for depression or suicidal thoughts. Discontinue Qsymia if symptoms develop. TTo report SUSPECTED ADVERSE REACTIONS, contact VIVUS Inc., at 1-888-998-4887 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS |
Phentermine hydrochloride orally disintegrating tablet c iv -suprenza™
| INDICATIONS AND USAGE: Suprenza is a sympathomimetic amine anorectic indicated as a short-term adjunct (a few weeks) in a regimen of weight reduction based on exercise, behavioral modification and caloric restriction in the management of exogenous obesity for patients with an initial body mass index greater than or equal to 30 kg/m2, or greater than or equal to 27 kg/m2 in the presence of other risk factors (e.g., controlled hypertension, diabetes, hyperlipidemia). The limited usefulness of agents of this class, including Suprenza, should be measured against possible risk factors inherent in their use. USE IN SPECIFIC POPULATIONS DOSAGE AND ADMINISTRATION: DOSAGE FORMS AND STRENGTHS: CONTRAINDICATIONS: WARNINGS AND PRECAUTIONS ADVERSE REACTIONS To report SUSPECTED ADVERSE REACTIONS, contact Akrimax Pharmaceuticals, LLC at 1-888-383-1733 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS |
Saxenda ® (liraglutide [rdna origin] injection)
| Drug UPDATES: SAXENDA ® (liraglutide [rDNA origin] injection), solution for subcutaneous use [Drug information / PDF] Dosing: Click (+) next to Dosage and Administration section (drug info link) Initial U.S. Approval: 2014 Mechanism of Action: Liraglutide is an acylated human glucagon-like peptide-1 (GLP-1) receptor agonist with 97% amino acid sequence homology to endogenous human GLP-1(7-37). Like endogenous GLP-1, liraglutide binds to and activates the GLP-1 receptor, a cell-surface receptor coupled to adenylyl cyclase activation through the stimulatory G-protein, Gs. Endogenous GLP-1 has a half-life of 1.5-2 minutes due to degradation by the ubiquitous endogenous enzymes, dipeptidyl peptidase 4 (DPP-4) and neutral endopeptidases (NEP). Unlike native GLP-1, liraglutide is stable against metabolic degradation by both peptidases and has a plasma half-life of 13 hours after subcutaneous administration. The pharmacokinetic profile of liraglutide, which makes it suitable for once-daily administration, is a result of self-association that delays absorption, plasma protein binding, and stability against metabolic degradation by DPP-4 and NEP. GLP-1 is a physiological regulator of appetite and calorie intake, and the GLP-1 receptor is present in several areas of the brain involved in appetite regulation. In animal studies, peripheral administration of liraglutide resulted in the presence of liraglutide in specific brain regions regulating appetite, including the hypothalamus. Although liraglutide activated neurons in brain regions known to regulate appetite, specific brain regions mediating the effects of liraglutide on appetite were not identified in rats. INDICATIONS AND USAGE: Limitations of Use: HOW SUPPLIED: Solution for subcutaneous injection, pre-filled, multi-dose pen that delivers doses of 0.6 mg, 1.2 mg, 1.8 mg, 2.4 mg or 3 mg (6 mg/mL, 3 mL) |
Contrave ® (naltrexone hcl and bupropion hcl)
| Drug UPDATES: CONTRAVE ® (naltrexone HCl and bupropion HCl) Dosing: Click (+) next to Dosage and Administration section (drug info link) Initial U.S. Approval: 2014 Mechanism of Action: CONTRAVE has two components: naltrexone, an opioid antagonist, and bupropion, a relatively weak inhibitor of the neuronal reuptake of dopamine and norepinephrine. Nonclinical studies suggest that naltrexone and bupropion have effects on two separate areas of the brain involved in the regulation of food intake: the hypothalamus (appetite regulatory center) and the mesolimbic dopamine circuit (reward system). The exact neurochemical effects of CONTRAVE leading to weight loss are not fully understood. INDICATIONS AND USAGE: 30 kg/m 2 or greater (obese) or Limitations of Use: HOW SUPPLIED: Extended-Release Tablets: 8 mg naltrexone HCl /90 mg bupropion HCl |
Reference(s)
National Institutes of Health, U.S. National Library of Medicine, DailyMed Database.
Provides access to the latest drug monographs submitted to the Food and Drug Administration (FDA). Please review the latest applicable package insert for additional information and possible updates. A local search option of this data can be found here.