VICTRELIS™ (boceprevir) Capsules
(description)
| These highlights do not include all the information needed to use VICTRELIS safely and effectively. See full prescribing information for VICTRELIS.
VICTRELIS™ (boceprevir) Capsules VICTRELIS (boceprevir) is an inhibitor of the hepatitis C virus (HCV) non-structural protein 3 (NS3) serine protease. Boceprevir has the following chemical name: (1R,5S)-N-[3-Amino-1-(cyclobutylmethyl)-2,3-dioxopropyl]-3-[2(S)-[[[(1,1-dimethylethyl)amino]carbonyl]amino]-3,3-dimethyl-1-oxobutyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexan-2(S)-carboxamide. The molecular formula is C27H45N5O5 and its molecular weight is 519.7. Boceprevir is manufactured as an approximately equal mixture of two diastereomers. Boceprevir is a white to off-white amorphous powder. It is freely soluble in methanol, ethanol and isopropanol and slightly soluble in water. |
Clinical pharmacology
| Mechanism of Action VICTRELIS is a direct acting antiviral drug against the hepatitis C virus. Microbiology |
Indications and usage
| INDICATIONS AND USAGE
VICTRELIS™ (boceprevir) is indicated for the treatment of chronic hepatitis C genotype 1 infection, in combination with peginterferon alfa and ribavirin, in adult patients (18 years and older) with compensated liver disease, including cirrhosis, who are previously untreated or who have failed previous interferon and ribavirin therapy. The following points should be considered when initiating VICTRELIS for treatment of chronic hepatitis C infection: VICTRELIS must not be used as monotherapy and should only be used in combination with peginterferon alfa and ribavirin. VICTRELIS efficacy has not been studied in patients who have previously failed therapy with a treatment regimen that includes VICTRELIS or other HCV NS3/4A protease inhibitors. VICTRELIS in combination with peginterferon alfa and ribavirin has not been studied in patients documented to be historical null responders (less than a 2-log10 HCV-RNA decline by treatment week 12) during prior therapy with peginterferon alfa and ribavirin. The clinical studies included subjects who were poorly interferon responsive. Subjects with less than 0.5-log10 HCV-RNA decline in viral load at Treatment Week 4 with peginterferon alfa plus ribavirin alone are predicted to have a null response (less than 2-log10 viral load decline at Treatment Week 12) to peginterferon alfa and ribavirin therapy. Poorly interferon responsive patients who were treated with VICTRELIS in combination with peginterferon alfa and ribavirin have a lower likelihood of achieving a sustained virologic response (SVR), and a higher rate of detection of resistance-associated substitutions upon treatment failure, compared to patients with a greater response to peginterferon alfa and ribavirin. USE IN SPECIFIC POPULATIONS Co-infection with Human Immunodeficiency Virus (HIV): Safety and efficacy have not been established in patients co-infected with HCV and HIV. Co-infection with Hepatitis B Virus (HBV): Safety and efficacy have not been studied in patients co-infected with HCV and HBV. Pediatrics: Safety and efficacy have not been studied in pediatric patients. |
Contraindications
| CONTRAINDICATIONS All contraindications to peginterferon alfa and ribavirin also apply since VICTRELIS must be administered with peginterferon alfa and ribavirin. Because ribavirin may cause birth defects and fetal death, boceprevir in combination with peginterferon alfa and ribavirin is contraindicated in pregnant women and in men whose female partners are pregnant. Coadministration with drugs that are highly dependent on CYP3A4/5 for clearance, and for which elevated plasma concentrations are associated with serious and/or life-threatening events. Potent CYP3A4/5 inducers where significantly reduced boceprevir plasma concentrations may be associated with reduced efficacy. |
Precautions
| WARNINGS AND PRECAUTIONS Use of VICTRELIS with Ribavirin and Peginterferon alfa: Ribavirin may cause birth defects and fetal death; avoid pregnancy in female patients and female partners of male patients. Patients must have a negative pregnancy test prior to therapy; use two or more forms of contraception, and have monthly pregnancy tests. Anemia - The addition of VICTRELIS to peginterferon alfa and ribavirin is associated with an additional decrease in hemoglobin concentrations compared with peginterferon alfa and ribavirin alone. Neutropenia - The addition of VICTRELIS to peginterferon alfa and ribavirin may result in worsening of neutropenia associated with peginterferon alfa and ribavirin therapy alone. DRUG INTERACTIONS |
Adverse reactions
| The most commonly reported adverse reactions (greater than 35% of subjects) in clinical trials in adult subjects receiving the combination of VICTRELIS with PegIntron and REBETOL were fatigue, anemia, nausea, headache and dysgeusia.
To report SUSPECTED ADVERSE REACTIONS, contact Schering Corporation, a subsidiary of Merck & Co., Inc., at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. |
Dosage and administration
| DOSAGE AND ADMINISTRATION
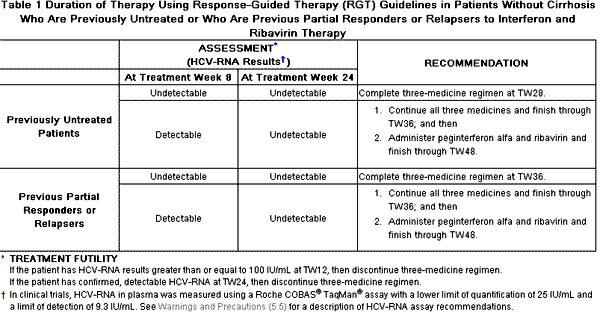
VICTRELIS must be administered in combination with peginterferon alfa and ribavirin. The dose of VICTRELIS is 800 mg (four 200-mg capsules) three times daily (every 7–9 hours) with food [a meal or light snack] (see Table 1). Refer to the peginterferon alfa and ribavirin Package Inserts for instructions on dosing. The following dosing recommendations differ for some subgroups from the dosing studied in the Phase 3 trials [see Clinical Studies (14)]. Response-Guided Therapy (RGT) is recommended for most individuals, but longer dosing is recommended in targeted subgroups (e.g., patients with cirrhosis). 2.1 VICTRELIS Combination Therapy: Patients Without Cirrhosis Who Are Previously Untreated or Who Are Previous Partial Responders or Relapsers to Interferon and Ribavirin Therapy Initiate therapy with peginterferon alfa and ribavirin for 4 weeks (Treatment Weeks 1–4).
Response-Guided Therapy was not studied in subjects who had less than a 2-log10 HCV-RNA decline by treatment week 12 during prior therapy with peginterferon alfa and ribavirin. If considered for treatment, these subjects should receive 4 weeks of peginterferon alfa and ribavirin followed by 44 weeks of VICTRELIS 800 mg orally three times daily (every 7–9 hours) in combination with peginterferon alfa and ribavirin. In addition, consideration should be given to treating previously untreated patients who are poorly interferon responsive (as determined at TW 4) with 4 weeks peginterferon alfa and ribavirin followed by 44 weeks of VICTRELIS 800 mg orally three times daily (every 7–9 hours) in combination with peginterferon alfa and ribavirin in order to maximize rates of SVR . 2.2 VICTRELIS Combination Therapy: Patients with Cirrhosis Patients with compensated cirrhosis should receive 4 weeks peginterferon alfa and ribavirin followed by 44 weeks VICTRELIS 800 mg (four 200-mg capsules) three times daily (every 7–9 hours) in combination with peginterferon alfa and ribavirin. 2.3 Dose Modification If a patient has a serious adverse reaction potentially related to peginterferon alfa and/or ribavirin, the peginterferon alfa and/or ribavirin dose should be reduced or discontinued. Refer to the peginterferon alfa and ribavirin Package Inserts for additional information about how to reduce and/or discontinue the peginterferon alfa and/or ribavirin dose. VICTRELIS must not be administered in the absence of peginterferon alfa and ribavirin. 2.4 Discontinuation of Dosing Based on Treatment Futility Discontinuation of therapy is recommended in all patients with 1) HCV-RNA levels of greater than or equal to 100 IU/mL at TW 12; or 2) confirmed detectable HCV-RNA levels at TW24. |
How supplied
| DOSAGE FORMS AND STRENGTHS Capsules: 200 mg How Supplied Storage and Handling |
Reference
| Package Insert data: Schering Corporation, a subsidiary of MERCK & CO., INC., Whitehouse Station, NJ 08889, USA U.S. Patent Nos. 7,012,066; 7,244,721 Trademarks depicted herein are the property of their respective owners. Copyright © 2011 Schering Corporation, a subsidiary of Merck & Co., Inc. All rights reserved. Issued: 05/11 34965510T |
Reference(s)
National Institutes of Health, U.S. National Library of Medicine, DailyMed Database.
Provides access to the latest drug monographs submitted to the Food and Drug Administration (FDA). Please review the latest applicable package insert for additional information and possible updates. A local search option of this data can be found here.