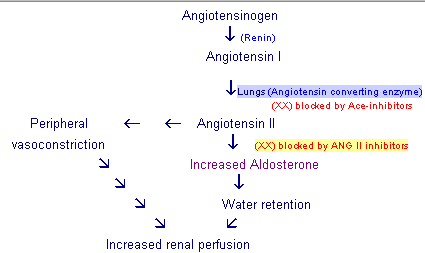
Angiotensin II inhibitors
Azilsartan medoxomil - (edarbi ®)
| Initial U.S. Approval: 2011 DESCRIPTION Edarbi (azilsartan medoxomil), a prodrug, is hydrolyzed to azilsartan in the gastrointestinal tract during absorption. Azilsartan is a selective AT1 subtype angiotensin II receptor antagonist. INDICATIONS AND USAGE DOSAGE AND ADMINISTRATION Edarbi may be administered with or without food. HOW SUPPLIED |
Candesartan (atacand ®)
| Supplied: 4 mg, 8 mg, 16 mg, 32 mg Dosing: Hypertension: Oral: Initial: 16 mg once daily. Range: 4 to 32 mg once daily. Dosage must be individualized. It can be administered once or twice daily with total daily doses ranging from 8-32 mg. CHF: Oral: Initial: 4 mg once daily. Double the dose at 2-week intervals, as tolerated; target dose: 32 mg. |
Eprosartan mesylate (teveten) ®
| Supplied: 400 mg, 600 mg Dosing: Hypertension: Oral: Usual initial dose is 600 mg once daily. Dosage must be individualized. Can administer once or twice daily with total daily doses of 400 to 800 mg. Limited clinical experience with doses greater than 800 mg. |
Irbesartan (avapro ®)
| Supplied: 75 mg, 150 mg, 300 mg Dosing: Hypertension: Oral: 150 mg once daily ... patients may be titrated to 300 mg once daily. Note: Starting dose in volume-depleted patients should be 75 mg. |
Losartan (cozaar ®)
| Dosing: Oral: Hypertension: Children 6-16 years: 0.7 mg/kg once daily (maximum: 50 mg/day); adjust dose based on response; doses >1.4 mg/kg (maximum: 100 mg) have not been studied Adults: Usual starting dose: 50 mg once daily; can be administered once or twice daily with total daily doses ranging from 25-100 mg Patients receiving diuretics or with intravascular volume depletion: Nephropathy in patients with type 2 diabetes and hypertension: Adults: Initial: 50 mg once daily; can be increased to 100 mg once daily based on blood pressure response Stroke reduction (HTN with LVH): Adults: 50 mg once daily (maximum daily dose: 100 mg); may be used in combination with a thiazide diuretic Dosing adjustment in renal impairment : Dosing adjustment in hepatic impairment : Reduce the initial dose to 25 mg/day; divide dosage intervals into two. Supplied: 25 mg, 50 mg, 100 mg |
Olmesartan (benicar ®)
| Supplied: 5 mg, 20 mg, 40 mg Dosing: Hypertension: Oral: Initial: Usual starting dose is 20 mg once daily. If initial response is inadequate, may be increased to 40 mg once daily after 2 weeks. Consider lower starting dose in patients with possible volume deficits. |
Telmisartan (micardis ®)
| Supplied: 20 mg, 40 mg, 80 mg Dosing: Hypertension: Oral: Initial: 40 mg once daily. Usual maintenance dose range: 20 to 80 mg per day. Patients with volume depletion should be initiated on the lower dosage with close supervision. |
Valsartan (diovan ®)
| Supplied: 40 mg, 80 mg, 160 mg, 320 mg Dosing: Hypertension: Initial: 80 mg or 160 mg once daily (in patients who are not volume depleted). Dose may be increased to achieve desired effect ... maximum recommended dose: 320 mg per day. CHF: Initial: 40 mg twice daily. Titrate dose to 80 to 160 mg twice daily, as tolerated. Maximum daily dose: 320 mg. |
Entresto™- sacubitril and valsartan tablet
| Drug UPDATES: ENTRESTO™- sacubitril and valsartan tablet [Drug information / PDF] Dosing: Click (+) next to Dosage and Administration section (drug info link) Initial U.S. Approval: 2015 Mechanism of Action: INDICATIONS AND USAGE: HOW SUPPLIED: |
Reference(s)
National Institutes of Health, U.S. National Library of Medicine, DailyMed Database.
Provides access to the latest drug monographs submitted to the Food and Drug Administration (FDA). Please review the latest applicable package insert for additional information and possible updates. A local search option of this data can be found here.