ZEMDRI ™ (plazomicin) injection |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Usual Diluents
|
||||||||||||||||||||||||
| NS, LR | ||||||||||||||||||||||||
Standard Dilutions [Amt of drug] [Infusion vol] [Infusion rate]
|
||||||||||||||||||||||||
| [15 mg/kg ] [50 ml total volume] [30 minutes]
Renal dosing: Additional info. Based on the wide range of compatible concentrations, keeping the total volume at 50 mL appears unnecessary: Supplied: ZEMDRI injection 500 mg/10 mL (50 mg/mL)
|
||||||||||||||||||||||||
WARNINGS ASSOCIATED WITH ZEMDRI (plazomicin)
|
||||||||||||||||||||||||
| See warnings and precautions below.
WARNING: NEPHROTOXICITY, OTOTOXICITY, NEUROMUSCULAR BLOCKADE and FETAL HARM
|
||||||||||||||||||||||||
DESCRIPTION
|
||||||||||||||||||||||||
| Description: ZEMDRI contains plazomicin sulfate, a semi-synthetic aminoglycoside antibacterial derived from sisomicin. The chemical name of plazomicin sulfate is (2"R,3"R,4"R,5"R)-2"-[(1S,2S,3R,4S,6R)-4-amino-6-[(2'"S)-4'"-amino-2'"-hydroxybutanamido)amino]-3-[(2'S,3'R)-3'-amino-6'-((2-hydroxyethylamino)methyl)-3',4'-dihydro-2H-pyran-2'-yloxy]-2-hydroxycyclohexyloxy]-5''-methyl-4''-(methylamino)tetrahydro-2H-pyran-3'',5''-diol sulfate. Plazomicin sulfate contains a theoretical 2.5 molar equivalents of sulfate relative to the freebase, based on complete protonation. The molecular weight of plazomicin sulfate is calculated based on 1:2.5 stoichiometry. The corresponding empirical formula is C25H48N6O10∙2.5 H2SO4 (plazomicin sulfate) and the molecular weight of the plazomicin sulfate salt is 837.89 g/mol and the molecular weight of the freebase is 592.69 g/mol.ZEMDRI injection 500 mg/10 mL is a sterile, clear, colorless-to-yellow liquid for 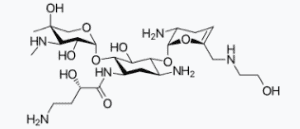 intravenous administration supplied in 10-mL single-dose Type 1 glass vials. Each vial contains plazomicin sulfate equivalent to 500 mg plazomicin freebase at a concentration of 50 mg/mL adjusted to pH 6.5. Each vial also contains Water for Injection and sodium hydroxide for pH adjustment. This sterile, nonpyrogenic solution is formulated without preservatives. |
||||||||||||||||||||||||
CLINICAL PHARMACOLOGY:
|
||||||||||||||||||||||||
| Mechanism of Action:
Plazomicin is an aminoglycoside that acts by binding to bacterial 30S ribosomal subunit, thereby inhibiting protein synthesis. Plazomicin has concentration-dependent bactericidal activity as measured by time kill studies. In vitro studies demonstrated a plazomicin post-antibiotic effect ranging from 0.2 to 2.6 hours at 2x MIC against Enterobacteriaceae. |
||||||||||||||||||||||||
INDICATIONS AND USAGE
|
||||||||||||||||||||||||
| INDICATIONS AND USAGE::
1.1 Complicated Urinary Tract Infections (cUTI), including Pyelonephritis ZEMDRI is indicated in patients 18 years of age or older for the treatment of complicated urinary tract infections (cUTI), including pyelonephritis caused by the following susceptible microorganism(s): Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Enterobacter cloacae. As only limited clinical safety and efficacy data for ZEMDRI are currently available, reserve ZEMDRI for use in cUTI patients who have limited or no alternative treatment options [see Clinical Studies (14.1)]. 1.2 Usage To reduce the development of drug-resistant bacteria and maintain the effectiveness of ZEMDRI and other antibacterial drugs, ZEMDRI should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy. |
||||||||||||||||||||||||
CONTRAINDICATIONS OF ZEMDRI
|
||||||||||||||||||||||||
| Contraindications::
ZEMDRI is contraindicated in patients with known hypersensitivity to any aminoglycoside [see Warnings and Precautions (5.5)]. |
||||||||||||||||||||||||
PRECAUTIONS
|
||||||||||||||||||||||||
WARNINGS AND PRECAUTIONS:
|
||||||||||||||||||||||||
ADVERSE REACTIONS
|
||||||||||||||||||||||||
| ADVERSE REACTIONS:
See PACKAGE INSERT for PATIENT COUNSELING INFORMATION and Medication Guide. Drug information |
||||||||||||||||||||||||
ZEMDRI (plazomicin) DOSAGE AND ADMINISTRATION
|
||||||||||||||||||||||||
| DOSAGE AND ADMINISTRATION:
2.1 Recommended Dosage The recommended dosage regimen of ZEMDRI is 15 mg/kg administered every 24 hours by intravenous (IV) infusion over 30 minutes in patients 18 years of age or older and with creatinine clearance (CLcr) greater than or equal to 90 mL/min (Table 1). The duration of therapy should be guided by the severity of infection and the patient's clinical status for up to 7 days. During treatment, dosage adjustments may be required based on change in renal function [see Dosage and Administration (2.3, 2.4)]. Table 1: Dosage Regimen of ZEMDRI in Adults With CLcr * Greater Than or Equal to 90 mL/min
2.2 Monitoring of Renal Function Assess creatinine clearance in all patients prior to initiating therapy and daily during therapy with ZEMDRI [see Dosage and Administration (2.3),Warnings and Precautions (5.1) and Use in Specific Populations (8.6)]. 2.3 Dosage in Adult Patients With Renal Impairment The recommended initial dosage regimen of ZEMDRI in adult patients with CLcr greater than or equal to 15 and less than 90 mL/min, estimated by the Cockcroft-Gault formula, is described in Table 2. Patients with CLcr greater than or equal to 15 and less than 90 mL/min receiving ZEMDRI may require subsequent dosage adjustments based on change in renal function and/or Therapeutic Drug Monitoring (TDM) as appropriate [see Dosage and Administration (2.4)]. Table 2: Dosage Regimen of ZEMDRI in Adults With CLcr Less Than 90 mL/min
There is insufficient information to recommend a dosage regimen in patients with CLcr less than 15 mL/min or on renal replacement therapy, including hemodialysis or continuous renal replacement therapy. 2.4 TDM in cUTI Patients With Renal Impairment 2.5 Preparation of Diluted Solutions of ZEMDRI ZEMDRI does not contain preservatives. Aseptic technique must be followed in preparing the infusion solution. Discard unused portion of the ZEMDRI vial. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. 2.6 Stability of ZEMDRI Solution in Intravenous Fluids
2.7 Drug Compatibility Compatibility of ZEMDRI for administration with other drugs has not been established. ZEMDRI should not be mixed with other drugs or physically added to solutions containing other drugs. Other medications should not be infused simultaneously with ZEMDRI through the same IV line. |
||||||||||||||||||||||||
HOW ZEMDRI (plazomicin) IS SUPPLIED
|
||||||||||||||||||||||||
| DOSAGE FORMS AND STRENGTHS::
ZEMDRI injection 500 mg/10 mL (50 mg/mL) is a sterile, clear, colorless to yellow solution supplied in a single-dose vial. Each single-dose vial contains plazomicin sulfate equivalent to 500 mg plazomicin freebase. |
||||||||||||||||||||||||
Storage and Stability
|
||||||||||||||||||||||||
| 16.1 How Supplied
ZEMDRI injection 500 mg/10 mL (50 mg/mL) is supplied in single-dose, 10-mL vials fitted with flip-off seals with royal blue polypropylene buttons as a clear, colorless to yellow, sterile solution. Each vial contains plazomicin sulfate equivalent to 500 mg plazomicin freebase at a concentration of 50 mg/mL plazomicin in Water for Injection. Each vial contains sodium hydroxide for pH adjustment to 6.5. The solution may become yellow in color; this does not indicate a decrease in potency.
16.2 Storage and Handling Store ZEMDRI injection 500 mg/10 mL (50 mg/mL) refrigerated at 2°C to 8°C (36°F to 46°F). Store ZEMDRI injection 500 mg/10 mL (50 mg/mL) refrigerated at 2°C to 8°C (36°F to 46°F). Manufactured for: NDC 71045-010-02 ZEMDRI™ 10 (10 mL) Single-dose vials For Intravenous Infusion Only Revised: 6/2018 |
||||||||||||||||||||||||

