TOBRAMYCIN |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The authors make no claims of the accuracy of the information contained herein; and these suggested doses and/or guidelines are not a substitute for clinical judgment. Neither GlobalRPh Inc. nor any other party involved in the preparation of this document shall be liable for any special, consequential, or exemplary damages resulting in whole or part from any user's use of or reliance upon this material. PLEASE READ THE DISCLAIMER CAREFULLY BEFORE ACCESSING OR USING THIS SITE. BY ACCESSING OR USING THIS SITE, YOU AGREE TO BE BOUND BY THE TERMS AND CONDITIONS SET FORTH IN THE DISCLAIMER. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Usual Diluents |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NS, D5W | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Standard Dilutions [Amount of drug] [Infusion volume] [Infusion rate] |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| [0 to 40 mg] [50 ml] [30 min]
[> 40 mg] [100 ml] [30-60 min] |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stability / Miscellaneous |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EXP: Tobramycin solution -IVPB: 24 hrs (RT) /48 hrs (REF) Reconstituted powder for injection: After reconstitution, the solution should be kept in a refrigerator and used within 96 hours. If kept at room temperature, the solution must be used within 24 hours.
Label: Refrigerate. DOSAGE AND ADMINISTRATION Administration for Patients with Normal Renal Function Adults with Life-Threatening Infections: Up to 5 mg/kg/day may be administered in 3 or 4 equal doses (see Table 1). The dosage should be reduced to 3 mg/kg/day as soon as clinically indicated. To prevent increased toxicity due to excessive blood levels, dosage should not exceed 5 mg/kg/day unless serum levels are monitored.
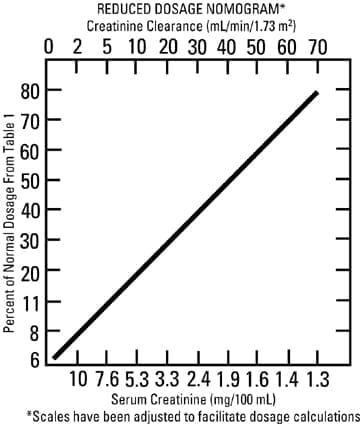
Children: 6 to 7.5 mg/kg/day in 3 or 4 equally divided doses (2 to 2.5 mg/kg every 8 hours or 1.5 to 1.89 mg/kg every 6 hours). It is desirable to limit treatment to a short term. The usual duration of treatment is 7 to 10 days. A longer course of therapy may be necessary in difficult and complicated infections. In such cases, monitoring of renal, auditory, and vestibular functions is advised, because neurotoxicity is more likely to occur when treatment is extended longer than ten days. Administration for Patients with Impaired Renal Function Following a loading dose of 1 mg/kg, subsequent dosage in these patients must be adjusted, either with reduced doses administered at 8-hour intervals or with normal doses given at prolonged intervals. Both of these methods are suggested as guides to be used when serum levels of tobramycin cannot be measured directly. They are based on either the creatinine clearance level or the serum creatinine of the patient, because these values correlate with the half-life of tobramycin. The dosage schedules derived from either method should be used in conjunction with careful clinical and laboratory observations of the patient and should be modified as necessary. Neither method should be used when dialysis is being performed. Reduced dosage at 8-hour intervals When the creatinine clearance rate is 70 mL or less per minute or when the serum creatinine value is known, the amount of the reduced dose can be determined by multiplying the normal dose from Table 1 by the percent of normal dose from the accompanying nomogram. An alternate rough guide for determining reduced dosage at 8-hour intervals (for patients whose steady-state serum creatinine values are known) is to divide the normally recommended dose by the patient’s serum creatinine.
Normal dosage at prolonged intervals If the creatinine clearance rate is not available and the patient’s condition is stable, a dosage frequency in hours for the dosage given in Table 1 can be determined by multiplying the patient’s serum creatinine by 6. Alternatively: Dosing interval in renal impairment: I.M., I.V.: High-dose therapy: Interval may be extended (eg, every 48 hours) in patients with moderate renal impairment (Clcr 30-59 mL/minute) and/or adjusted based on serum level determinations. Hemodialysis: Dialyzable; 30% removal of aminoglycosides occurs during 4 hours of HD - administer dose after dialysis and follow levels -------------------------------------------------------------------------------- Dosage in Obese Patients Intramuscular Administration Intravenous Administration Tobramycin sulfate should not be physically premixed with other drugs but should be administered separately according to the recommended dose and route. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. HOW SUPPLIED Storage APOTHECON® 1135942 Source: [package insert] |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||