Acetylcysteine - Acetadote ® |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The authors make no claims of the accuracy of the information contained herein; and these suggested doses and/or guidelines are not a substitute for clinical judgment. Neither GlobalRPh Inc. nor any other party involved in the preparation of this document shall be liable for any special, consequential, or exemplary damages resulting in whole or part from any user's use of or reliance upon this material. PLEASE READ THE DISCLAIMER CAREFULLY BEFORE ACCESSING OR USING THIS SITE. BY ACCESSING OR USING THIS SITE, YOU AGREE TO BE BOUND BY THE TERMS AND CONDITIONS SET FORTH IN THE DISCLAIMER. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Usual Diluents |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D5W
Additional comments: Acetadote is hyperosmolar (2600 mOsm/L) and is compatible with:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Standard Dilutions [Amount of drug] [Infusion volume] [Infusion rate] |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Acetaminophen overdose: Patients ≥40 kg (See comments below): Loading Dose: 150 mg/kg (maximum of 15 grams) in 200 mL of diluent administered over 60 min Dose 2: 50 mg/kg (maximum of 5 grams) in 500 mL of diluent administered over 4 hr Dose 3: 100 mg/kg (maximum of 10 grams) in 1000 mL of diluent administered over 16 hr
Patients >20 - <40 kg (See comments below): Patients ≤20 kg (See comments below): |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stability / Miscellaneous |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stability data:Stability Refrigerated: Notes: Single dose vial, Note: The color of acetylcysteine injection may turn from essentially
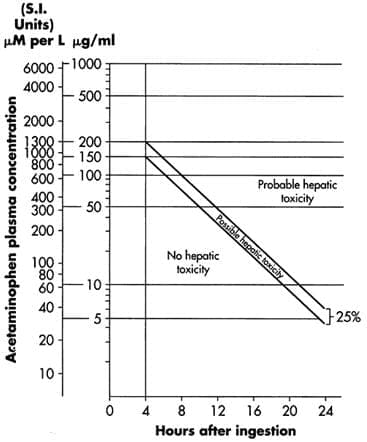
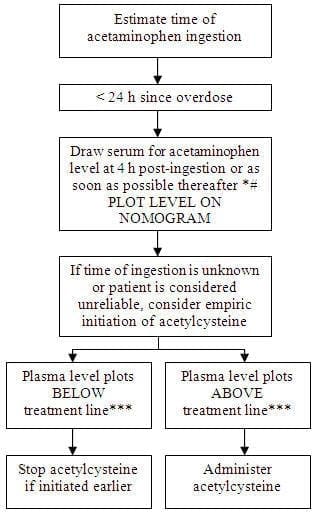
Do not use previously opened vials for IV administration. CLINICAL PHARMACOLOGY Acetaminophen Overdose: Acetylcysteine I.V. Treatment: INDICATIONS AND USAGE On admission for suspected acetaminophen overdose, a serum blood sample should be drawn at least 4 hours after ingestion to determine the acetaminophen level and will serve as a basis for determining the need for treatment with acetylcysteine. If the patient presents after 4 hours post-ingestion, the serum acetaminophen sample should be determined immediately. Acetadote should be administered within 8 hours from acetaminophen ingestion for maximal protection against hepatic injury for patients whose serum acetaminophen levels fall above the "possible" toxicity line on the Rumack-Matthew nomogram (line connecting 150 mcg/mL at 4 hours with 37.5 mcg/mL at 12 hours); [see Acetaminophen Assays – Interpretation and Methodology (1.1, 1.2)]. If the time of ingestion is unknown, or the serum acetaminophen level is not available, cannot be interpreted, or is not available within the 8 hour time interval from acetaminophen ingestion, Acetadote should be administered immediately if 24 hours or less have elapsed from the reported time of ingestion of an overdose of acetaminophen, regardless of the quantity reported to have been ingested. The aspartate aminotransferase (AST, SGOT), alanine aminotranferase (ALT, SGPT), bilirubin, prothrombin time, creatinine, blood urea nitrogen (BUN), blood glucose, and electrolytes also should be determined in order to monitor hepatic and renal function and electrolyte and fluid balance. NOTE: The critical ingestion-treatment interval for maximal protection against severe hepatic injury is between 0 – 8 hours. Efficacy diminishes progressively after 8 hours and treatment initiation between 15 and 24 hours post-ingestion of acetaminophen yields limited efficacy. However, it does not appear to worsen the condition of patients and it should not be withheld, since the reported time of ingestion may not be correct. Acetaminophen Assays Interpretation and Methodology – Acute Ingestion Interpretation of Acetaminophen Assays
Estimating Potential for Hepatotoxicity: The following depiction of the Rumack-Matthew nomogram has been developed to estimate the probability that plasma levels in relation to intervals post-ingestion will result in hepatotoxicity. Figure 1. Rumack-Matthew Nomogram: Plasma or Serum Acetaminophen Concentration vs. Time Post Acetaminophen Ingestion (Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics. 1975;55:871-876 and Rumack BH, Peterson RC, Kock GG, Amara IA. Acetaminophen overdose. 662 cases with evaluation of oral acetylcysteine treatment. Arch Intern Med. 1981;141:380-385).
Acetaminophen Assays Interpretation and Methodology – Repeated
# With an extended-release preparation, an acetaminophen level drawn less than 8 hours post-ingestion may be misleading. Draw a second level at 4 to 6 hours after the initial level. If either falls above the toxicity line, acetylcysteine treatment should be initiated. ***Acetylcysteine may be withheld until acetaminophen assay results are available as long as initiation of treatment is not delayed beyond 8 hours post-ingestion. If more than 8 hours post-ingestion, start acetylcysteine treatment immediately. DOSAGE AND ADMINISTRATION Administration Instructions (Three-Bag Method: Loading, Second and Third Dose) Loading Dose: 150 mg/kg (maximum of 15 grams) in 200 mL of diluent administered over 60 min Second Dose: 50 mg/kg (maximum of 5 grams) in 500 mL of diluent administered over 4 hr Third Dose: 100 mg/kg (maximum of 10 grams) in 1000 mL of diluent administered over 16 hr
1. Acetadote is hyperosmolar (2600 mOsm/L) and is compatible with 5% Dextrose (D5W), ½ Normal Saline (0.45% Sodium Chloride Injection, ½ NS), and Water for Injection (WFI). The total volume administered should be adjusted for patients less than 40 kg and for those requiring fluid restriction: Patients >20 - <40 kg (Table 2):
1. Acetadote is hyperosmolar (2600 mOsm/L) and is compatible with 5% Dextrose (D5W), ½ Normal Saline (0.45% Sodium Chloride Injection, ½ NS), and Water for Injection (WFI). Patients ≤20 kg (Table 3):
1. Acetadote is hyperosmolar (2600 mOsm/L) and is compatible with 5% Dextrose (D5W), ½ Normal Saline (0.45% Sodium Chloride Injection, ½ NS), and Water for Injection (WFI). Stability: Stability studies indicate that the diluted solution is stable for 24 hours at controlled room temperature. Note: The color of Acetadote may turn from essentially colorless to a slight pink or purple once the stopper is punctured. The color change does not affect the quality of the product. Renal Impairment Hepatic Impairment DOSAGE FORMS AND STRENGTHS CONTRAINDICATIONS WARNINGS AND PRECAUTIONS Anaphylactoid Reactions Acute flushing and erythema of the skin may occur in patients receiving acetylcysteine intravenously. These reactions usually occur 30 to 60 minutes after initiating the infusion and often resolve spontaneously despite continued infusion of acetylcysteine. Anaphylactoid reactions (defined as the occurrence of an acute hypersensitivity reaction during acetylcysteine administration including rash, hypotension, wheezing, and/or shortness of breath) have been observed in patients receiving I.V. acetylcysteine for acetaminophen overdose and occurred soon after initiation of the infusion [see Adverse Reactions (6.1)]. If a reaction to acetylcysteine involves more than simply flushing and erythema of the skin, it should be treated as an anaphylactoid reaction. This usually entails administering antihistaminic drugs and in severe cases may require administration of epinephrine. In addition, the acetylcysteine infusion may be interrupted until treatment of the anaphylactoid symptoms has been initiated and then carefully restarted. If the anaphylactoid reaction returns upon reinitiation of treatment or increases in severity, intravenous acetylcysteine should be discontinued and alternative patient management should be considered. Monitoring patients with asthma Volume Adjustment: Patients <40kg and Requiring Fluid Restriction For specific treatment information regarding the clinical management of acetaminophen overdose, please contact your regional poison center at 1-800-222-1222, or alternatively, a special health professional assistance line for acetaminophen overdose at 1-800-525-6115. USE IN SPECIFIC POPULATIONS Pregnancy There are no adequate and well-controlled studies of Acetadote in pregnant women. However, limited case reports of pregnant women exposed to acetylcysteine during various trimesters did not report any adverse maternal, fetal or neonatal outcomes. There are published reports on four pregnant women with acetaminophen toxicity, who were treated with oral or intravenous acetylcysteine at the time of delivery. Acetylcysteine crossed the placenta and was measurable following delivery in serum and cord blood of three viable infants and in cardiac blood of a fourth infant at autopsy (22 weeks gestational age who died 3 hours after birth). No adverse sequelae developed in the three viable infants. All mothers recovered and none of the infants had evidence of acetaminophen poisoning. Reproductive and developmental toxicity studies performed in rats at oral doses up to 6.7 times the recommended human intravenous dose and in rabbits at doses up to 3.3 times the recommended human intravenous dose revealed no evidence of impaired fertility or embryofetal toxicity [see package insert for Reproductive and Developmental Toxicology (13.3)]. Nursing mothers Pediatric use Geriatric use OVERDOSAGE HOW SUPPLIED/STORAGE AND HANDLING 30 mL vials, carton of 4 (NDC 66220-107-30) Note: The color of Acetadote may turn from essentially colorless to a slight pink or purple once the stopper is punctured. The color change does not affect the quality of the product. The stopper in the Acetadote vial is formulated with a synthetic base-polymer and does not contain Natural Rubber Latex, Dry Natural Rubber, or blends of Natural Rubber. Storage Manufactured for: *Sections or subsections omitted from the Full Prescribing Information are not listed. PRINCIPAL DISPLAY PANEL - Label 30 mL 200mg/mL (6g/30mL) CUMBERLAND® PHARMACEUTICALS Source: Package insert
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||