Evkeeza
1 INDICATIONS AND USAGE
EVKEEZA is indicated as an adjunct to other low-density lipoprotein-cholesterol (LDL-C) lowering therapies for the treatment of adult and pediatric patients, aged 12 years and older, with homozygous familial hypercholesterolemia (HoFH).
2 DOSAGE AND ADMINISTRATION
- The recommended dose of EVKEEZA is 15 mg/kg administered by intravenous (IV) infusion once monthly (every 4 weeks). (2.1)
- See the Full Prescribing Information for preparation instructions for the intravenous infusion. (2.2)
- Administer the diluted solution via IV infusion over 60 minutes through an IV line containing a sterile, in-line or add-on, 0.2 micron to 5 micron filter. (2.3)
- Do not mix other medications with EVKEEZA or administer other medications concomitantly via the same infusion line. (2.3)
- The rate of infusion may be slowed, interrupted or discontinued if the patient develops any signs of adverse reactions, including infusion or hypersensitivity reactions. (2.3).
2.1 Recommended Dosage
- The recommended dose of EVKEEZA is 15 mg/kg administered by intravenous (IV) infusion over 60 minutes once monthly (every 4 weeks).
- If a dose of EVKEEZA is missed, administer as soon as possible. Thereafter, EVKEEZA should be scheduled monthly from the date of the last dose.
- Assess LDL-C when clinically appropriate. The LDL-lowering effect of EVKEEZA may be measured as early as 2 weeks after initiation.
2.2 Preparation Instructions for Intravenous Infusion
- Calculate the dose (mg), total volume (mL) of EVKEEZA required, and the number of vials required based on the patient's current body weight.
- Visually inspect the solution for cloudiness, discoloration, and particulate matter prior to administration. EVKEEZA is a clear to slightly opalescent, colorless to pale-yellow solution. Do not administer if the solution is cloudy or discolored or contains particulate matter.
- EVKEEZA vials are single-dose containers and do not contain a preservative. Observe aseptic technique when preparing EVKEEZA.
- Do not shake the vial. Withdraw the required volume from the vial(s) of EVKEEZA and transfer into an IV infusion bag containing a maximum volume of 250 mL of 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP. Mix the diluted solution by gentle inversion; do not shake.
- The final concentration of the diluted solution should be between 0.5 mg/mL and 20 mg/mL depending on the patient's current body weight.
- Administer the diluted solution immediately after preparation and discard any unused portion left in the vial.
- If not used immediately, store the diluted solution refrigerated at 2 °C to 8 °C (36 °F to 46 °F) for no more than 24 hours from the time of preparation OR at room temperature up to 25 °C (77 °F) for no more than 6 hours from the time of infusion preparation to the end of the infusion. Do not freeze the diluted solution.
2.3 Administration Instructions for Intravenous Infusion
- If refrigerated, allow the diluted solution to come to room temperature prior to administration.
- Administer EVKEEZA diluted solution via IV infusion over 60 minutes through an IV line containing a sterile, in-line or add-on, 0.2-micron to 5-micron filter.
- Do not mix other medications with EVKEEZA or administer other medications concomitantly via the same infusion line.
- The rate of infusion may be slowed, interrupted or discontinued if the patient develops any signs of adverse reactions, including infusion or hypersensitivity reactions [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
- EVKEEZA can be administered without regard to the timing of lipoprotein apheresis.
3 DOSAGE FORMS AND STRENGTHS
EVKEEZA is a clear to slightly opalescent, colorless to pale yellow solution available as follows:
- Injection: 345 mg/2.3 mL (150 mg/mL) and 1,200 mg/8 mL (150 mg/mL) in single-dose vials.
4 CONTRAINDICATIONS
EVKEEZA is contraindicated in patients with a history of serious hypersensitivity reaction to evinacumab-dgnb or to any of the excipients in EVKEEZA. Serious hypersensitivity reactions, including anaphylaxis, have occurred [see Warnings and Precautions (5.1)].
5 WARNINGS AND PRECAUTIONS
- Serious Hypersensitivity Reactions: Have occurred with EVKEEZA in clinical trials. If a serious hypersensitivity reaction occurs, discontinue EVKEEZA, treat according to standard-of-care and monitor until signs and symptoms resolve. (5.1)
- Embryo-Fetal Toxicity: EVKEEZA may cause fetal harm based on animal studies. Advise patients who may become pregnant of the risk to a fetus. Consider obtaining a pregnancy test prior to initiating treatment with EVKEEZA. Advise patients who may become pregnant to use contraception during treatment and for at least 5 months following the last dose. (5.2, 8.1, 8.3)
5.1 Serious Hypersensitivity Reactions
Serious hypersensitivity reactions have occurred with EVKEEZA. In clinical trials, 1 (1%) EVKEEZA-treated patient experienced anaphylaxis versus 0 (0%) patients who received placebo. If signs or symptoms of serious hypersensitivity reactions occur, discontinue EVKEEZA infusion, treat according to the standard-of-care, and monitor until signs and symptoms resolve. EVKEEZA is contraindicated in patients with a history of serious hypersensitivity reaction to evinacumab-dgnb [see Contraindications (4)].
5.2 Embryo-Fetal Toxicity
Based on the findings in animal reproduction studies, EVKEEZA may cause fetal harm when administered to pregnant patients. Administration of evinacumab to rabbits during organogenesis caused increases in fetal malformations at doses below the human exposure. Advise patients who may become pregnant of the risk to a fetus. Consider obtaining a pregnancy test prior to initiating treatment with EVKEEZA. Advise patients who may become pregnant to use effective contraception during treatment with EVKEEZA and for at least 5 months following the last dose of EVKEEZA [see Use in Specific Populations (8.1, 8.3)].
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Safety data are based on pooled results from two randomized, double-blind, placebo-controlled trials that included 81 patients treated with EVKEEZA. The mean age of EVKEEZA-treated patients was 48 years (range: 15 to 75 years), 52% were women, 5% were Hispanic, 82% were White, 7% Asian, 3% Black, and 9% Other. Forty-four (54%) EVKEEZA-treated patients had HoFH. Patients received EVKEEZA as add-on therapy to other lipid-lowering therapies, including maximally tolerated statin, ezetimibe, PCSK9 inhibitors, lomitapide, and apheresis.
Adverse reactions led to discontinuation of treatment in 2 (2%) patients treated with EVKEEZA, including 1 case of anaphylaxis, and 1 (2%) patient who received placebo. The most common adverse reactions (reported in greater than 3% of EVKEEZA-treated patients and more frequently than in placebo) are shown in Table 1.
| Adverse Reactions | Placebo (N = 54) % |
EVKEEZA (N = 81) % |
|---|---|---|
| Nasopharyngitis | 13% | 16% |
| Influenza like illness | 6% | 7% |
| Dizziness | 0% | 6% |
| Rhinorrhea | 0% | 5% |
| Nausea | 2% | 5% |
| Pain in extremity | 0% | 4% |
| Asthenia | 0% | 4% |
Other adverse reactions occurring in less than 3% of patients treated with EVKEEZA and greater than placebo included constipation, upper respiratory tract infection, nasal congestion, and abdominal pain.
Transient, mild to moderate decreases in diastolic blood pressure and increases in heart rate occurred in clinical trials of EVKEEZA infusion but did not require intervention and resolved post-infusion.
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to EVKEEZA in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
No patients developed treatment-emergent antibodies to EVKEEZA.
8 USE IN SPECIFIC POPULATIONS
Risk Summary
Based on data from animal reproduction studies, EVKEEZA may cause fetal harm when administered to pregnant patients. Available human data are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Evinacumab-dgnb is a human IgG4 monoclonal antibody [see Description (11)], and human IgG is known to cross the placental barrier; therefore, evinacumab-dgnb has the potential to be transmitted from the mother to the developing fetus.
Subcutaneous administration of evinacumab-dgnb to pregnant rabbits during the period of organogenesis resulted in fetal malformations (domed head, hydrocephalus, and flexed limbs) at doses below the maximum recommended human dose (MRHD). No adverse embryofetal effects were observed with subcutaneous administration of evinacumab-dgnb to pregnant rats during the period of organogenesis at doses below the MRHD. Measurable evinacumab-dgnb serum concentrations were observed in fetal rabbit and rat sera at birth, indicating that evinacumab-dgnb, like other IgG antibodies, crosses the placental barrier (see Data). Advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population are unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
If a patient becomes pregnant while receiving EVKEEZA, healthcare providers should report EVKEEZA exposure by calling 1-833-385-3392.
8.1 Pregnancy
Risk Summary
Based on data from animal reproduction studies, EVKEEZA may cause fetal harm when administered to pregnant patients. Available human data are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Evinacumab-dgnb is a human IgG4 monoclonal antibody [see Description (11)], and human IgG is known to cross the placental barrier; therefore, evinacumab-dgnb has the potential to be transmitted from the mother to the developing fetus.
Subcutaneous administration of evinacumab-dgnb to pregnant rabbits during the period of organogenesis resulted in fetal malformations (domed head, hydrocephalus, and flexed limbs) at doses below the maximum recommended human dose (MRHD). No adverse embryofetal effects were observed with subcutaneous administration of evinacumab-dgnb to pregnant rats during the period of organogenesis at doses below the MRHD. Measurable evinacumab-dgnb serum concentrations were observed in fetal rabbit and rat sera at birth, indicating that evinacumab-dgnb, like other IgG antibodies, crosses the placental barrier (see Data). Advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population are unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
If a patient becomes pregnant while receiving EVKEEZA, healthcare providers should report EVKEEZA exposure by calling 1-833-385-3392.
8.2 Lactation
Risk Summary
There are no data on the presence of evinacumab-dgnb in human milk or animal milk, the effects on the breastfed infant, or the effects on milk production. Maternal IgG is known to be present in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed infant to evinacumab-dgnb are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for EVKEEZA and any potential adverse effects on the breastfed infant from EVKEEZA or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Consider pregnancy testing in patients who may become pregnant prior to starting treatment with EVKEEZA [see Warnings and Precautions (5.2) and Use in Specific Populations (8.1)].
8.4 Pediatric Use
The safety and effectiveness of EVKEEZA as an adjunct to other LDL-C-lowering therapies for the treatment of HoFH have been established in pediatric patients aged 12 years and older. Use of EVKEEZA for this indication is supported by evidence from adequate and well-controlled trials in adults with additional efficacy and safety data in pediatric patients aged 12 years and older [see Adverse Reactions (6.1) and Clinical Studies (14)].
The safety and effectiveness of EVKEEZA have not been established in pediatric patients with HoFH who are younger than 12 years old.
8.5 Geriatric Use
Clinical studies of EVKEEZA did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients.
11 DESCRIPTION
Evinacumab-dgnb is an angiopoietin-like protein 3 (ANGPTL3) inhibitor monoclonal antibody (IgG4 isotype) produced by recombinant DNA technology in Chinese hamster ovary (CHO) cell suspension culture. Evinacumab-dgnb has an approximate molecular weight of 146 kDa.
EVKEEZA (evinacumab-dgnb) injection is a sterile, preservative-free solution for intravenous use. The solution is clear to slightly opalescent, colorless to pale-yellow, and free from visible particles.
Each vial contains 345 mg/2.3 mL or 1,200 mg/8 mL. Each mL contains 150 mg of evinacumab-dgnb, and L-arginine hydrochloride (14.8 mg), L-histidine (0.74 mg), L-histidine monohydrochloride monohydrate (1.1 mg), L-proline (30 mg), polysorbate 80 (1 mg) and Water for Injection, USP. The pH is 6.
12 CLINICAL PHARMACOLOGY
Evinacumab-dgnb is a recombinant human monoclonal antibody that binds to and inhibits ANGPTL3. ANGPTL3 is a member of the angiopoietin-like protein family that is expressed primarily in the liver and plays a role in the regulation of lipid metabolism by inhibiting lipoprotein lipase (LPL) and endothelial lipase (EL). Evinacumab-dgnb inhibition of ANGPTL3 leads to reduction in LDL-C, HDL-C, and triglycerides (TG). Evinacumab-dgnb reduces LDL-C independent of the presence of LDL receptor (LDLR) by promoting very low-density lipoprotein (VLDL) processing and clearance upstream of LDL formation. Evinacumab-dgnb blockade of ANGPTL3 lowers TG and HDL-C by rescuing LPL and EL activities, respectively.
12.1 Mechanism of Action
Evinacumab-dgnb is a recombinant human monoclonal antibody that binds to and inhibits ANGPTL3. ANGPTL3 is a member of the angiopoietin-like protein family that is expressed primarily in the liver and plays a role in the regulation of lipid metabolism by inhibiting lipoprotein lipase (LPL) and endothelial lipase (EL). Evinacumab-dgnb inhibition of ANGPTL3 leads to reduction in LDL-C, HDL-C, and triglycerides (TG). Evinacumab-dgnb reduces LDL-C independent of the presence of LDL receptor (LDLR) by promoting very low-density lipoprotein (VLDL) processing and clearance upstream of LDL formation. Evinacumab-dgnb blockade of ANGPTL3 lowers TG and HDL-C by rescuing LPL and EL activities, respectively.
12.2 Pharmacodynamics
Administration of evinacumab-dgnb in HoFH patients resulted in reductions in LDL-C, total cholesterol (TC), HDL-C, apolipoprotein B and TG [see Clinical Studies (14)].
12.3 Pharmacokinetics
The pharmacokinetic parameters described in this section are presented following administration of evinacumab-dgnb 15 mg/kg intravenously every 4 weeks, unless otherwise specified.
Steady-state is reached after 4 doses, and the accumulation ratio is 2. According to population pharmacokinetic modeling, the mean (standard deviation) steady-state trough concentration is 241 (96.5) mg/L, whereas the mean (standard deviation) Cmax at the end of infusion is 689 (157) mg/L. Due to non-linear clearance, a 4.3-fold increase in area under the concentration-time curve at steady-state (AUCtau.ss) for a 3-fold increase in evinacumab-dgnb dose up to 15 mg/kg IV every 4 weeks was predicted in patients with HoFH.
13 NONCLINICAL TOXICOLOGY
Carcinogenesis and Mutagenesis
Carcinogenicity studies have not been conducted with evinacumab-dgnb. The mutagenic potential of evinacumab-dgnb has not been evaluated; however, monoclonal antibodies are not expected to alter DNA or chromosomes.
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis and Mutagenesis
Carcinogenicity studies have not been conducted with evinacumab-dgnb. The mutagenic potential of evinacumab-dgnb has not been evaluated; however, monoclonal antibodies are not expected to alter DNA or chromosomes.
14 CLINICAL STUDIES
Study ELIPSE-HoFH (NCT03399786) was a multicenter, double-blind, randomized, placebo-controlled trial evaluating the efficacy and safety of EVKEEZA compared to placebo in 65 patients with HoFH. During the 24-week, double-blind treatment period, 43 patients were randomized to receive EVKEEZA 15 mg/kg IV every 4 weeks and 22 patients to receive placebo. After the double-blind treatment period, 64 of 65 patients entered a 24-week open-label extension period in which all patients received EVKEEZA 15 mg/kg IV every 4 weeks.
Patients were on a background of other lipid-lowering therapies, including maximally tolerated statins, ezetimibe, PCSK9 inhibitor antibodies, lomitapide, and lipoprotein apheresis. Enrolment was stratified by apheresis status and geographical region. The diagnosis of HoFH was determined by genetic testing or by the presence of the following clinical criteria: history of an untreated total cholesterol (TC) >500 mg/dL and either xanthoma before 10 years of age or evidence of TC >250 mg/dL in both parents. In this trial, 40% (26 of 65) patients had limited LDL receptor (LDLR) function, defined by either <15% receptor function by in vitro assays or by genetic variants likely to result in minimal to no LDLR function by mutation analysis.
The mean LDL-C at baseline was 255 mg/dL. In patients with limited LDLR function, the mean LDL-C at baseline was 307 mg/dL. At baseline, 94% of patients were on statins, 75% on ezetimibe, 77% on a PCSK9 inhibitor antibody, 22% on lomitapide, and 34% were receiving lipoprotein apheresis. The mean age at baseline was 42 years (range 12 to 75) with 12% ≥65 years old; 54% women, 3% Hispanic, 74% White, 15% Asian, 3% Black, and 8% Other or not reported.
The primary efficacy endpoint was percent change in LDL-C from baseline to Week 24. At Week 24, the least squares (LS) mean treatment difference between EVKEEZA and placebo in mean percent change in LDL-C from baseline was −49% (95% confidence interval: −65% to −33%; p <0.0001). After 24 weeks of open-label EVKEEZA treatment (Week 24 to Week 48), the observed LDL-C reduction from baseline was similar in patients who crossed over from placebo to EVKEEZA and was maintained in patients who remained on EVKEEZA for 48 weeks. For efficacy results see Table 2.
| LDL-C | ApoB | Non-HDL-C | TC | TG |
HDL-C |
|
|---|---|---|---|---|---|---|
| Abbreviations: HoFH=homozygous familial hypercholesterolemia, ITT=intent-to-treat, LS mean=least squares mean, N=number of randomized patients, CI=confidence interval | ||||||
|
Baseline (mean), mg/dL (N=65) |
255 | 171 | 278 | 322 | 124 | 44 |
|
LS Mean: EVKEEZA (N = 43) |
-47% | -41% | -50% | -47% | -55% | -30% One subject in the placebo group discontinued the study before Week 24. The treatment difference and 95% confidence interval (CI) were estimated using a mixed model repeated measures analysis. |
|
LS Mean: Placebo (N = 22) |
+2% | -5% | +2% | +1% | -5% | +1% |
|
LS Mean Difference from Placebo (95% CI) |
-49% (-65 to -33) |
-37% (-49 to -25) |
-52% (-65 to -39) |
-48% (-59 to -38) |
-50% (-66 to -35) |
- |
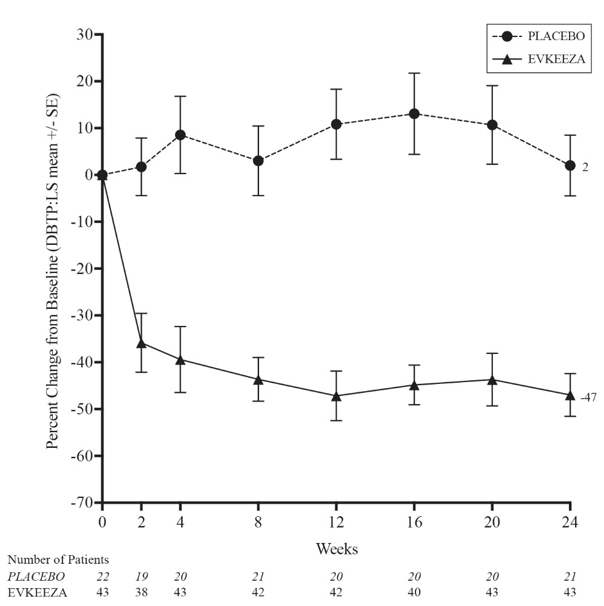
The LS mean LDL-C percent changes over time are presented in Figure 1.
Figure 1: Calculated LDL-C LS Mean Percent Change from Baseline Over Time Through Week 24 in Study ELIPSE-HoFH
| Abbreviations: LS mean=least squares mean, HoFH=homozygous familial hypercholesterolemia, DBTP=double-blind treatment period, SE=standard error |
|
|
At Week 24, the observed reduction in LDL-C with EVKEEZA was similar across predefined subgroups, including age, sex, limited LDLR activity, concomitant treatment with lipoprotein apheresis, and concomitant background lipid-lowering medications (statins, ezetimibe, PCSK9 inhibitor antibodies, and lomitapide).
16 HOW SUPPLIED/STORAGE AND HANDLING
EVKEEZA (evinacumab-dgnb) injection is a clear to slightly opalescent, colorless to pale yellow solution. It is supplied as one single-dose vial per carton.
- 345 mg/2.3 mL (150 mg/mL) NDC 61755-013-01
- 1,200 mg/8 mL (150 mg/mL) NDC 61755-010-01
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
PRINCIPAL DISPLAY PANEL - 345 mg/2.3 mL Vial Carton
NDC 61755-013-01
Rx only
Evkeeza™
(evinacumab-dgnb)
Injection
345 mg/2.3 mL (150 mg/mL)
For Intravenous Infusion after Dilution
Single-Dose Vial – Discard Unused Portion
Must dilute before use.
Do not use vial if seal is broken or missing.
Do not use after expiration.
Store in the original carton
to protect from light.

PRINCIPAL DISPLAY PANEL - 1200 mg/8 mL Vial Carton
NDC 61755-010-01
Rx only
Evkeeza™
(evinacumab-dgnb)
Injection
1200 mg/8 mL (150 mg/mL)
For Intravenous Infusion after Dilution
Single-Dose Vial – Discard Unused Portion
Must dilute before use.
Do not use vial if seal is broken or missing.
Do not use after expiration.
Store in the original carton
to protect from light.