WARNINGS
SERIOUS INFUSION REACTIONS, PROLONGED AND SEVERE CYTOPENIAS, and SEVERE CUTANEOUS AND MUCOCUTANEOUS REACTIONS
Serious Infusion Reactions: Deaths have occurred within 24 hours of rituximab infusion, an essential component of the Zevalin therapeutic regimen. These fatalities were associated with hypoxia, pulmonary infiltrates, acute respiratory distress syndrome, myocardial infarction, ventricular fibrillation, or cardiogenic shock. Most (80%) fatalities occurred with the first rituximab infusion [see PACKAGE INSERT FOR Warnings and Precautions (5.1) and Adverse Reactions (6.1)]. Discontinue rituximab, In-111 Zevalin, and Y-90 Zevalin infusions in patients who develop severe infusion reactions.
Prolonged and Severe Cytopenias: Y-90 Zevalin administration results in severe and prolonged cytopenias in most patients. Do not administer the Zevalin therapeutic regimen to patients with ≥ 25% lymphoma marrow involvement and/or impaired bone marrow reserve [see PACKAGE INSERT FOR Warnings and Precautions (5.2) and Adverse Reactions (6.1)].
Severe Cutaneous and Mucocutaneous Reactions: Severe cutaneous and mucocutaneous reactions, some fatal, can occur with the Zevalin therapeutic regimen. Discontinue rituximab, In-111 Zevalin, and Y-90 Zevalin infusions in patients experiencing severe cutaneous or mucocutaneous reactions [see PACKAGE INSERT FOR Warnings and Precautions (5.3) and Adverse Reactions (6.3)].
Dosing: The dose of Y-90 Zevalin should not exceed 32.0 mCi (1184 MBq). Do not administer Y-90 Zevalin to patients with altered biodistribution as determined by imaging with In-111 Zevalin [see Dosage and Administration (2.2)]. |
DESCRIPTION
Zevalin (ibritumomab tiuxetan) is the immunoconjugate resulting from a stable thiourea covalent bond between the monoclonal antibody ibritumomab and the linker-chelator tiuxetan [N-[2-bis(carboxymethyl)amino]-3-(p-isothiocyanatophenyl)-propyl]-[N-[2-bis(carboxymethyl)amino]-2-(methyl)-ethyl]glycine. This linker-chelator provides a high affinity, conformationally restricted chelation site for Indium-111 or Yttrium-90. The approximate molecular weight of ibritumomab tiuxetan is 148 kD. The antibody moiety of Zevalin is ibritumomab, a murine IgG1 kappa monoclonal antibody directed against the CD20 antigen.
Ibritumomab tiuxetan is a clear, colorless, sterile, pyrogen-free, preservative-free solution that may contain translucent particles. Each single-use vial includes 3.2 mg of ibritumomab tiuxetan in 2 mL of 0.9% Sodium Chloride.
CLINICAL PHARMACOLOGY
Mechanism of Action
Ibritumomab tiuxetan binds specifically to the CD20 antigen (human B-lymphocyte-restricted differentiation antigen, Bp35). The apparent affinity (KD) of ibritumomab tiuxetan for the CD20 antigen ranges between approximately 14 to 18 nM. The CD20 antigen is expressed on pre-B and mature B lymphocytes and on > 90% of B-cell non-Hodgkin’s lymphomas (NHL). The CD20 antigen is not shed from the cell surface and does not internalize upon antibody binding.
The chelate tiuxetan, which tightly binds In-111 or Y-90, is covalently linked to ibritumomab. The beta emission from Y-90 induces cellular damage by the formation of free radicals in the target and neighboring cells.
Ibritumomab tiuxetan binding was observed in vitro on lymphoid cells of the bone marrow, lymph node, thymus, red and white pulp of the spleen, and lymphoid follicles of the tonsil, as well as lymphoid nodules of other organs such as the large and small intestines.
Pharmacodynamics
In clinical studies, administration of the Zevalin therapeutic regimen resulted in sustained depletion of circulating B cells. At four weeks, the median number of circulating B cells was zero (range, 0-1084/mm3). B-cell recovery began at approximately 12 weeks following treatment, and the median level of B cells was within the normal range (32 to 341/mm3) by 9 months after treatment. Median serum levels of IgG and IgA remained within the normal range throughout the period of B-cell depletion. Median IgM serum levels dropped below normal (median 49 mg/dL, range 13-3990 mg/dL) after treatment and recovered to normal values by 6-months post therapy.
Pharmacokinetics
Pharmacokinetic and biodistribution studies were performed using In-111 Zevalin (5 mCi [185 MBq] In-111, 1.6 mg ibritumomab tiuxetan). In an early study designed to assess the need for pre-administration of unlabeled antibody, only 18% of known sites of disease were imaged when In-111 Zevalin was administered without unlabeled ibritumomab. When preceded by unlabeled ibritumomab (1.0 mg/kg or 2.5 mg/kg), In-111 Zevalin detected 56% and 92% of known disease sites, respectively. These studies were conducted with a Zevalin therapeutic regimen that included unlabeled ibritumomab.
In pharmacokinetic studies of patients receiving the Zevalin therapeutic regimen, the mean effective half-life for Y-90 activity in blood was 30 hours, and the mean area under the fraction of injected activity (FIA) vs. time curve in blood was 39 hours. Over 7 days, a median of 7.2% of the injected activity was excreted in urine.
INDICATIONS AND USAGE
1.1 Relapsed or Refractory, Low-grade or Follicular NHL
Zevalin is indicated for the treatment of relapsed or refractory, low-grade or follicular B-cell non-Hodgkin's lymphoma (NHL).
1.2 Previously Untreated Follicular NHL
Zevalin is indicated for the treatment of previously untreated follicular NHL in patients who achieve a partial or complete response to first-line chemotherapy.
2. DOSAGE AND ADMINISTRATION
Recommended Dosing Schedule: Administer the Zevalin therapeutic regimen as outlined in Section 2.1. Initiate the Zevalin therapeutic regimen following recovery of platelet counts to ≥150,000/mm3 at least 6 weeks, but no more than 12 weeks, following the last dose of first-line chemotherapy.
2.1 Overview of Dosing Schedule

2.2 Zevalin Therapeutic Regimen Dosage and Administration
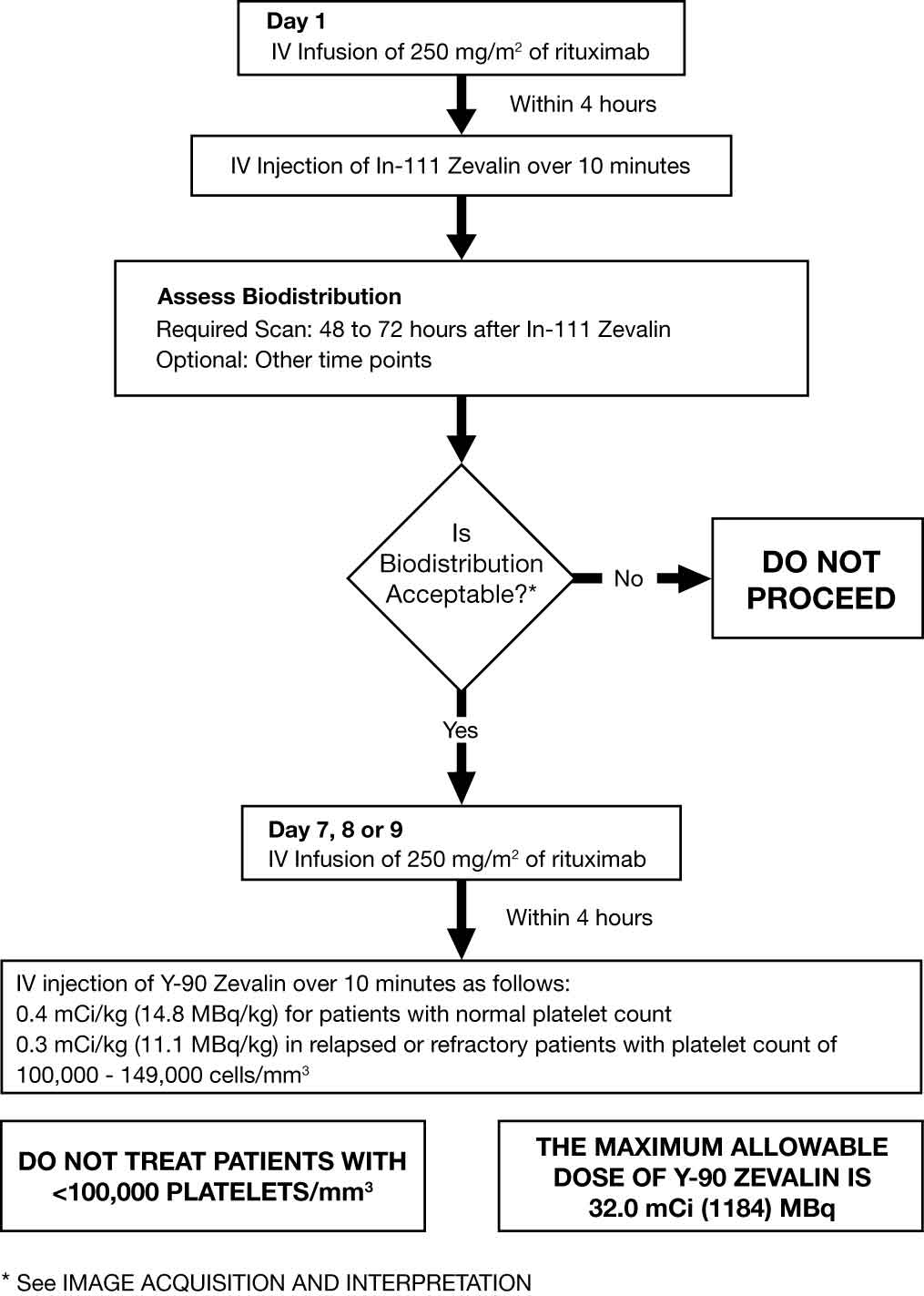
Day 1: Premedicate with acetaminophen 650 mg orally and diphenhydramine 50 mg orally prior to rituximab infusion. Administer rituximab 250 mg/m2 intravenously at an initial rate of 50 mg/hr. In the absence of infusion reactions, escalate the infusion rate in 50 mg/hr increments every 30 minutes to a maximum of 400 mg/hr. Do not mix or dilute rituximab with other drugs. Immediately stop the rituximab infusion for serious infusion reactions and discontinue the Zevalin therapeutic regimen [see Boxed Warning and Warnings and Precautions (5.1)]. Temporarily slow or interrupt the rituximab infusion for less severe infusion reactions. If symptoms improve, continue the infusion at one-half the previous rate. Administer 5 mCi In-111 Zevalin over 10 minutes as an intravenous injection within 4 hours following completion of the rituximab infusion. Use a 0.22 micron low-protein-binding in-line filter between the syringe and the infusion port. After injection, flush the line with at least 10 mL of normal saline Day 7, 8 or 9:
Verify that expected biodistribution is present [see Dosage and Administration (2.5)].
Premedicate with acetaminophen 650 mg orally and diphenhydramine 50 mg orally prior to rituximab infusion. Administer rituximab 250 mg/m2 intravenously at an initial rate of 100 mg/hr. Increase rate by 100 mg/hr increments at 30 minute intervals, to a maximum of 400 mg/hr, as tolerated. If infusion reactions occurred during rituximab infusion on Day 1 of treatment, administer rituximab at an initial rate of 50 mg/hr and escalate the infusion rate in 50 mg/hr increments every 30 minutes to a maximum of 400 mg/hr. Administer Y-90 Zevalin injection through a free flowing intravenous line within 4 hours following completion of rituximab infusion. Use a 0.22 micron low-protein-binding in-line filter between the syringe and the infusion port. After injection, flush the line with at least 10 mL of normal saline. If platelet count ≥150,000/mm3, administer Y-90 Zevalin over 10 minutes as an intravenous injection at a dose of Y-90 0.4 mCi per kg (14.8 MBq per kg) actual body weight. If platelet count 100,000-149,000/mm3, in relapsed or refractory patients, administer Y-90 Zevalin over 10 minutes as an intravenous injection at a dose of Y-90 0.3 mCi per kg (11.1 MBq per kg) actual body weight. Do not administer more than 32 mCi (1184 MBq) Y-90 Zevalin dose regardless of the patient’s body weight. Monitor patients closely for evidence of extravasation during the injection of Y-90 Zevalin. Immediately stop infusion and restart in another limb if any signs or symptoms of extravasation occur [see PACKAGE INSERT FOR Warnings and Precautions (5.7)].
2.3 Directions for Preparation of Radiolabeled In-111 and Y-90 Zevalin Doses
Two separate and distinctly-labeled kits are required for preparation of Indium-111 (In-111) Zevalin and Yttrium-90 (Y-90) Zevalin. Follow the detailed instructions for the preparation of radiolabeled Zevalin [see Dosage and Administration (2.4)]. The procedures are different for the preparation of In-111 Zevalin and of Y-90 Zevalin.
Directions for Preparation of Radiolabeled In-111 Zevalin Dose
Required materials not supplied in the kit: Indium-111 Chloride Sterile Solution (In-111 Chloride) from GE Healthcare, or Mallinckrodt/Covidien Three sterile 1 mL plastic syringes One sterile 3 mL plastic syringe Two sterile 10 mL plastic syringes with 18-20 G needles Instant thin-layer chromatographic (ITLC) silica gel strips 0.9% Sodium Chloride aqueous solution for the chromatography solvent Developing chamber for chromatography Suitable radioactivity counting apparatus Filter, 0.22 micrometer, low-protein-binding Appropriate lead shielding for reaction vial and syringe for In-111 Method: Allow contents of the refrigerated In-111 Zevalin kit (Zevalin vial, 50 mM sodium acetate vial, formulation buffer vial, and empty reaction vial) to reach room temperature. Place the empty reaction vial in an appropriate lead shield. Determine the amount of each component needed: Calculate volume of In-111 Chloride equivalent to 5.5 mCi based on the activity concentration of the In-111 Chloride stock. The volume of 50 mM Sodium Acetate solution needed is 1.2 times the volume of In-111 Chloride solution determined in step 3.a, above. Calculate volume of formulation buffer needed to bring the reaction vial contents to a final volume of 10 mL. Transfer the calculated volume of 50 mM of Sodium Acetate to the empty reaction vial. Coat the entire inner surface of the reaction vial by gentle inversion or rolling. Transfer 5.5 mCi of In-111 Chloride to the reaction vial using a lead shielded syringe. Mix the two solutions by gentle inversion or rolling. Transfer 1 mL of Zevalin (ibritumomab tiuxetan) to the reaction vial. Do not shake or agitate the vial contents. Allow the labeling reaction to proceed at room temperature for 30 minutes. A shorter or longer reaction time may adversely alter the final labeled product. Immediately after the 30-minute incubation period, transfer the calculated volume of formulation buffer from step 3.c. to the reaction vial. Gently add the formulation buffer down the side of the reaction vial. If necessary, withdraw an equal volume of air to normalize pressure. Measure the final product for total activity using a radioactivity calibration system suitable for the measurement of In-111. Using supplied labels, record the date and time of preparation, total activity and volume, date and time of expiration, and affix these labels to the shielded reaction vial container. Patient Dose: Calculate the volume required for an In-111 Zevalin dose of 5 mCi. Withdraw the required volume from the reaction vial into a sterile syringe. Assay the syringe in a dose calibrator suitable for the measurement of In-111. Using the supplied labels, record patient identifier, total activity and volume and the date and time of expiration, and affix these labels to the syringe and shielded unit dose container. Determine Radiochemical Purity [see Dosage and Administration (2.4)]. Store Indium-111 Zevalin at 2-8°C (36-46°F) until use and administer within 12 hours of radiolabeling. Immediately prior to administration, assay the syringe and contents using an appropriate radioactivity calibration system. Directions for Preparation of Radiolabeled Y-90 Zevalin Dose
Required materials not supplied in the kit: Yttrium-90 Chloride Sterile Solution Three sterile 1 mL plastic syringes One sterile 3 mL plastic syringe Two sterile 10 mL plastic syringes with 18-20 G needles ITLC silica gel strips 0.9% Sodium Chloride aqueous solution for the chromatography solvent Developing chamber for chromatography Suitable radioactivity counting apparatus Filter, 0.22 micrometer, low-protein-binding Appropriate acrylic shielding for reaction vial and syringe for Y-90 Method: Allow contents of the refrigerated Y-90 Zevalin kit (Zevalin vial, 50 mM sodium acetate vial, and formulation buffer vial) to reach room temperature. Place the empty reaction vial in an appropriate acrylic shield. Determine the amount of each component needed: Calculate volume of Y-90 Chloride equivalent to 40 mCi based on the activity concentration of the Y-90 Chloride stock. The volume of 50 mM Sodium Acetate solution needed is 1.2 times the volume of Y-90 Chloride solution determined in step 3.a, above. Calculate the volume of formulation buffer needed to bring the reaction vial contents to a final volume of 10 mL. Transfer the calculated volume of 50 mM Sodium Acetate to the empty reaction vial. Coat the entire inner surface of the reaction vial by gentle inversion or rolling. Transfer 40 mCi of Y-90 Chloride to the reaction vial using an acrylic shielded syringe. Mix the two solutions by gentle inversion or rolling. Transfer 1.3 mL of Zevalin (ibritumomab tiuxetan) to the reaction vial. Do not shake or agitate the vial contents. Allow the labeling reaction to proceed at room temperature for 5 minutes. A shorter or longer reaction time may adversely alter the final labeled product. Immediately after the 5-minute incubation period, transfer the calculated volume of formulation buffer from step 3.c. to the reaction vial. Gently add the formulation buffer down the side of the reaction vial. If necessary, withdraw an equal volume of air to normalize pressure. Measure the final product for total activity using a radioactivity calibration system suitable for the measurement of Y-90. Using the supplied labels, record the date and time of preparation, the total activity and volume, and the date and time of expiration, and affix these labels to the shielded reaction vial container. Patient Dose: Calculate the volume required for a Y-90 Zevalin dose [see Dosage and Administration (2.2)]. Withdraw the required volume from the reaction vial. Assay the syringe in the dose calibrator suitable for the measurement of Y-90. The measured dose must be within 10% of the prescribed dose of Y-90 Zevalin and must not exceed 32 mCi (1184 MBq). Using the supplied labels, record the patient identifier, total activity and volume and the date and time of expiration, and affix these labels to the syringe and shielded unit dose container. Determine Radiochemical Purity [see Dosage and Administration (2.4)]. Store Yttrium-90 Zevalin at 2-8°C (36-46°F) until use and administer within 8 hours of radiolabeling. Immediately prior to administration, assay the syringe and contents using a radioactivity calibration system suitable for the measurement of Y-90
2.4 Procedure for Determining Radiochemical Purity
Use the following procedures for radiolabeling both In-111 Zevalin and
Y-90 Zevalin Place a small drop of either In-111 Zevalin or Y-90 Zevalin at the origin of an ITLC silica gel strip. Place the ITLC silica gel strip into a chromatography chamber with the origin at the bottom and the solvent front at the top. Allow the solvent (0.9% NaCl) to migrate at least 5 cm from the bottom of the strip. Remove the strip from the chamber and cut the strip in half. Count each half of the ITLC silica gel strip for one minute (CPM) with a suitable counting apparatus. Calculate the percent RCP as follows  Repeat the ITLC procedure if the radiochemical purity is <95%. If repeat testing confirms that radiochemical purity is <95%, do not administer the In-111 or Y-90 Zevalin dos 2.5 Image Acquisition and Interpretation of Biodistribution Repeat the ITLC procedure if the radiochemical purity is <95%. If repeat testing confirms that radiochemical purity is <95%, do not administer the In-111 or Y-90 Zevalin dos 2.5 Image Acquisition and Interpretation of Biodistribution
Assess the biodistribution of In-111 Zevalin by a visual evaluation of whole body planar view anterior and posterior gamma images obtained at 48 - 72 hours after injection. Images at additional time points may be necessary to resolve ambiguities. Acquire whole body anterior/posterior planar images using a large field-of-view gamma camera and medium energy collimators. Suggested gamma camera settings: 256 x 1024 matrix; dual energy photopeaks set at 172 and 247 keV; 15% symmetric window; scan speed of 10 cm/min for the 48-72 hour scan, and 7-10 cm/min for subsequent scans.
Expected Biodistribution Activity in the blood pool areas (heart, abdomen, neck, and extremities) may be faintly visible. Moderately high to high uptake in normal liver and spleen. Moderately low or very low uptake in normal kidneys, urinary bladder, and normal (uninvolved) bowel. Non-fixed areas within the bowel lumen that change position with time; delayed imaging as described above may be necessary to confirm gastrointestinal clearance. Focal fixed areas of uptake in the bowel wall (localization to lymphoid aggregates in bowel wall). Tumor uptake may be visualized however tumor visualization on the In-111 Zevalin scan is not required for Y-90 Zevalin therapy.
Altered Biodistribution
The criteria for altered biodistribution are met if any of the following is detected on visual inspection of the required gamma images: Intense localization of radiotracer in the liver and spleen and bone marrow indicative of reticuloendothelial system uptake. Increased uptake in normal organs (not involved by tumor) such as: Diffuse uptake in normal lung more intense than the liver. Kidneys have greater intensity than the liver on the posterior view. Fixed areas (unchanged with time) of uptake in the normal bowel greater than uptake in the liver. In less than 0.5% of patients receiving In-111 Zevalin, prominent bone marrow uptake was observed, characterized by clear visualization of the long bones and ribs Consider bone marrow involvement by lymphoma, increased marrow activity due to recent hematopoietic growth factor administration, and increased reticuloendothelial uptake in patients with HAMA and HACA, as possible causes of prominent bone marrow uptake. Re-assess biodistribution after correction of underlying factors.
[SEE PACKAGE INSERT FOR ADDITIONAL INFO ....]
3. DOSAGE FORMS AND STRENGTHS
3.2 mg ibritumomab tiuxetan per 2 mL, single-use vial.
4. CONTRAINDICATIONS
None.
HOW SUPPLIED/STORAGE AND HANDLING
There are two kits necessary for preparation of the Zevalin therapeutic regimen: one for preparation of In-111 radiolabeled Zevalin (NDC 68152-104-04) and one for preparation of Y-90 radiolabeled Zevalin (NDC 68152-103-03). The contents of all vials are sterile, pyrogen-free, contain no preservatives, and are not radioactive. Each kit contains four identification labels and the following four vials One (1) Zevalin vial containing 3.2 mg ibritumomab tiuxetan in 2 mL 0.9% Sodium Chloride as a clear, colorless solution. One (1) 50 mM Sodium Acetate Vial containing 13.6 mg Sodium Acetate trihydrate in 2 mL Water for Injection, USP as a clear, colorless solution. One (1) Formulation Buffer Vial containing 750 mg Albumin (Human), 76 mg Sodium Chloride, 28 mg Sodium Phosphate Dibasic Dodecahydrate, 4 mg Pentetic Acid, 2 mg Potassium Phosphate Monobasic and 2 mg Potassium Chloride in 10 mL Water for Injection, pH 7.1 as a clear yellow to amber colored solution. One (1) empty Reaction Vial Indium-111 Chloride Sterile Solution (In-111 Chloride) must be ordered separately from either GE Healthcare, or Mallinckrodt/Covidien.
Yttrium-90 Chloride Sterile Solution is shipped directly from the supplier upon placement of an order for the Y-90 Zevalin kit.
Rituximab (Rituxan®, Biogen Idec and Genentech USA) must be ordered separately.
Storage
Store kits at 2-8°C (36-46°F). Do not freeze
|


 Repeat the ITLC procedure if the radiochemical purity is <95%. If repeat testing confirms that radiochemical purity is <95%, do not administer the In-111 or Y-90 Zevalin dos 2.5 Image Acquisition and Interpretation of Biodistribution
Repeat the ITLC procedure if the radiochemical purity is <95%. If repeat testing confirms that radiochemical purity is <95%, do not administer the In-111 or Y-90 Zevalin dos 2.5 Image Acquisition and Interpretation of Biodistribution