Oxaliplatin - Eloxatin® |
||||||||||
| The authors make no claims of the accuracy of the information contained herein; and these suggested doses and/or guidelines are not a substitute for clinical judgment. Neither GlobalRPh Inc. nor any other party involved in the preparation of this document shall be liable for any special, consequential, or exemplary damages resulting in whole or part from any user's use of or reliance upon this material. PLEASE READ THE DISCLAIMER CAREFULLY BEFORE ACCESSING OR USING THIS SITE. BY ACCESSING OR USING THIS SITE, YOU AGREE TO BE BOUND BY THE TERMS AND CONDITIONS SET FORTH IN THE DISCLAIMER. | ||||||||||
Usual Diluents |
||||||||||
| D5W | ||||||||||
Dilution Data |
||||||||||
Preparation of Infusion Solution Stability: 2] Concentrated solution preparation: The solution must be further diluted in an infusion solution of 250-500 mL of 5% Dextrose Injection, USP. After dilution with 250-500 mL of 5% Dextrose Injection, USP, the shelf life is 6 hours at room temperature [20-25°C (68-77°F)] or up to 24 hours under refrigeration [2-8°C (36-46°F)]. After final dilution, protection from light is not required. Storage (vials) - Store under normal lighting conditions at 20°-25°C (68°-77°F); excursions permitted to 15-30°C (59- 86°F) [see USP controlled room temperature]. Do not freeze. Oxaliplatin Injection is incompatible in solution with alkaline medications or media (such as basic solutions of 5-fluorouracil) and must not be mixed with these or administered simultaneously through the same infusion line. The infusion line should be flushed with 5% Dextrose Injection, USP prior to administration of any concomitant medication. Needles or intravenous administration sets containing aluminum parts that may come in contact with Oxaliplatin Injection should not be used for the preparation or mixing of the drug. Aluminum has been reported to cause degradation of platinum compounds. |
||||||||||
| Stability / Miscellaneous | ||||||||||
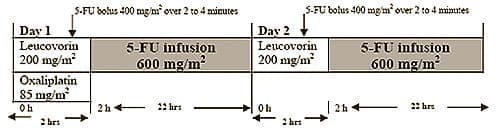
Administer Oxaliplatin Injection in combination with 5-fluorouracil/leucovorin every 2 weeks: Day 1: Oxaliplatin Injection 85 mg/m2 intravenous infusion in 250-500 mL 5% Dextrose Injection, USP and leucovorin 200 mg/m2 intravenous infusion in 5% Dextrose Injection, USP both given over 120 minutes at the same time in separate bags using a Y-line, followed by 5-fluorouracil 400 mg/m2 intravenous bolus given over 2 to 4 minutes, followed by 5-fluorouracil 600 mg/m2 intravenous infusion in 500 mL 5% Dextrose Injection, USP (recommended) as a 22-hour continuous infusion. Day 2: Leucovorin 200 mg/m2 intravenous infusion over 120 minutes followed by 5-fluorouracil 400 mg/m2 intravenous bolus given over 2 to 4 minutes followed by 5-fluorouracil 600 mg/m2 intravenous infusion in 500 mL 5% Dextrose Injection, USP (recommended) as a 22-hour continuous infusion. Reduce the dose of Oxaliplatin Injection to 75 mg/m² (adjuvant setting) or 65 mg/m² (advanced colorectal cancer): if there are persistent grade 2 neurosensory events that do not resolve. after recovery from grade 3/4 gastrointestinal toxicities (despite prophylactic treatment) or grade 4 neutropenia or grade 3/4 thrombocytopenia. Delay next dose until neutrophils ≥ 1.5 x 109/L and platelets ≥ 75 x 109/L. Discontinue Oxaliplatin Injection if there are persistent Grade 3 neurosensory events. Never reconstitute or prepare final dilution with a sodium chloride solution or other chloride-containing solutions.
1. INDICATIONS AND USAGE - adjuvant treatment of stage III colon cancer in patients who have undergone complete resection of the primary tumor. - treatment of advanced colorectal cancer. 2. DOSAGE AND ADMINISTRATION 2.1 Dosage Day 1: Oxaliplatin Injection 85 mg/m2 intravenous infusion in 250-500 mL 5% Dextrose injection, USP and leucovorin 200 mg/m2 intravenous infusion in 5% Dextrose Injection, USP both given over 120 minutes at the same time in separate bags using a Y-line, followed by 5-fluorouracil 400 mg/m2 intravenous bolus given over 2 to 4 minutes, followed by 5-fluorouracil 600 mg/m2 intravenous infusion in 500 mL 5% Dextrose Injection, USP (recommended) as a 22-hour continuous infusion. Day 2: Leucovorin 200 mg/m2 intravenous infusion over 120 minutes, followed by 5-fluorouracil 400 mg/m2 intravenous bolus given over 2 to 4 minutes, followed by 5-fluorouracil 600 mg/m2 intravenous infusion in 500 mL 5% Dextrose Injection, USP (recommended) as a 22-hour continuous infusion. Figure 1 The administration of Oxaliplatin Injection does not require prehydration. Premedication with antiemetics, including 5-HT3 blockers with or without dexamethasone, is recommended. 2.2 Dose Modification Recommendations Adjuvant Therapy in Patients with Stage III Colon Cancer: Neuropathy and other toxicities were graded using the NCI CTC scale version 1 [see Warnings and Precautions (5.2)]. For patients who experience persistent Grade 2 neurosensory events that do not resolve, a dose reduction of Oxaliplatin Injection to 75 mg/m2 should be considered. For patients with persistent Grade 3 neurosensory events, discontinuing therapy should be considered. The infusional 5-fluorouracil/leucovorin regimen need not be altered. A dose reduction of Oxaliplatin Injection to 75 mg/m2 and infusional 5-fluorouracil to 300 mg/m2 bolus and 500 mg/m2 22 hour infusion is recommended for patients after recovery from grade 3/4 gastrointestinal (despite prophylactic treatment) or grade 4 neutropenia or grade 3/4 thrombocytopenia. The next dose should be delayed until: neutrophils ≥ 1.5 x 109/L and platelets ≥ 75 x 109/L. Dose Modifications in Therapy in Previously Untreated and Previously Treated Patients with Advanced Colorectal Cancer: Neuropathy was graded using a study-specific neurotoxicity scale [see PACKAGE INSERT FOR Warnings and Precautions (5.2)]. Other toxicities were graded by the NCI CTC, Version 2.0. For patients who experience persistent Grade 2 neurosensory events that do not resolve, a dose reduction of Oxaliplatin Injection to 65 mg/m2 should be considered. For patients with persistent Grade 3 neurosensory events, discontinuing therapy should be considered. The 5-fluorouracil/leucovorin regimen need not be altered. A dose reduction of Oxaliplatin Injection to 65 mg/m2 and 5-FU by 20% (300 mg/m2 bolus and 500 mg/m2 22-hour infusion) is recommended for patients after recovery from grade 3/4 gastrointestinal (despite prophylactic treatment) or grade 4 neutropenia or grade 3/4 thrombocytopenia. The next dose should be delayed until: neutrophils ≥ 1.5 x 109/L and platelets ≥ 75 x 109/L. ----------------------------------------------------------------------- A final dilution must never be performed with a sodium chloride solution or other chloride-containing solutions. The solution must be further diluted in an infusion solution of 250-500 mL of 5% Dextrose Injection, USP. After dilution with 250-500 mL of 5% Dextrose Injection, USP, the shelf life is 6 hours at room temperature [20-25°C (68-77°F)] or up to 24 hours under refrigeration [2-8°C (36-46°F)]. After final dilution, protection from light is not required. Oxaliplatin Injection is incompatible in solution with alkaline medications or media (such as basic solutions of 5-fluorouracil) and must not be mixed with these or administered simultaneously through the same infusion line. The infusion line should be flushed with 5% Dextrose Injection, USP prior to administration of any concomitant medication. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration and discarded if present. Needles or intravenous administration sets containing aluminum parts that may come in contact with Oxaliplatin Injection should not be used for the preparation or mixing of the drug. Aluminum has been reported to cause degradation of platinum compounds. 3. DOSAGE FORMS AND STRENGTHS 4. CONTRAINDICATIONS HOW SUPPLIED/STORAGE AND HANDLING How Supplied NDC 61703-363-18: 50 mg/10 mL single use vial individually packaged in a carton. NDC 61703-363-22: 100 mg/20 mL single use vial individually packaged in a carton. Storage Handling and Disposal Procedures for the handling and disposal of anticancer drugs should be considered. Several guidelines on the subject have been published. There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
|
||||||||||
| Reference(s) | ||||||||||
| 1) [PACKAGE INSERT DATA] : OXALIPLATIN injection, solution. [Hospira Worldwide, Inc.] Lake Forest, IL 60045. Revision June 2009.
|
||||||||||
Eloxatin® (Oxaliplatin)