ANDEXXA- Andexanet alfa injection |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
Usual Diluents
|
|||||||||||||||||||||||||||||||||||
| (reconstituted solution is placed into an empty polyolefin or polyvinyl chloride IV bag with a volume of 250 mL or less.)
100 mg vials: Reconstitute the 100 mg vial of ANDEXXA with 10 mL of Sterile Water for Injection, USP (SWFI) Or 200 mg vials: Reconstitute the 200 mg vial of ANDEXXA with 20 mL of SWFI.
|
|||||||||||||||||||||||||||||||||||
Standard Dilutions [Amt of drug] [Infusion vol] [Infusion rate]
|
|||||||||||||||||||||||||||||||||||
| IV Bolus Preparation: Determine total number of vials required (see Table 1). 100 mg vials: Reconstitute the 100 mg vial of ANDEXXA with 10 mL of Sterile Water for Injection, USP (SWFI) Or 200 mg vials: Reconstitute the 200 mg vial of ANDEXXA with 20 mL of SWFI.Use a 20-mL (or larger) syringe and 20-gauge (or higher) needle. Slowly inject the SWFI, directing the solution onto the inside wall of the vial to minimize foaming. To reduce the total reconstitution time needed during preparation, reconstitute all required vials in succession.To ensure dissolution of the cake or powder, gently swirl each vial until complete dissolution of powder occurs. Do not shake; shaking could lead to foaming. Typical dissolution time for each vial is approximately three to five minutes. If dissolution is incomplete, discard the vial, and do not use the product. Upon reconstitution, the parenteral drug product should be inspected visually for particulate matter and discoloration prior to administration.Use 60-mL (or larger) syringe with a 20-gauge (or higher) needle to withdraw the reconstituted ANDEXXA solution from each of the vials until the required dosing volume is achieved. Note the total volume withdrawn into the syringe. Transfer the ANDEXXA solution from the syringe into an empty polyolefin or polyvinyl chloride IV bag with a volume of 250 mL or less. Discard the syringe and needle. Discard the vials, including any unused portion. Continuous IV Infusion Preparation
2.3 Administration
|
|||||||||||||||||||||||||||||||||||
WARNINGS
|
|||||||||||||||||||||||||||||||||||
See warnings and precautions below.
|
|||||||||||||||||||||||||||||||||||
DESCRIPTION OF ANDEXXA
|
|||||||||||||||||||||||||||||||||||
| Description:
ANDEXXA (coagulation factor Xa (recombinant), inactivated-zhzo) is a sterile, white to off-white lyophilized powder available in single-use vials. The Generation 2 product is produced using a modified Generation 1 manufacturing process. Each 100 mg vial delivers 100 mg of coagulation factor Xa formulated with the inactive ingredients tromethamine (Tris), L-arginine hydrochloride, sucrose (2% w/v), mannitol (5% w/v), and polysorbate 80 (0.01% w/v) at pH 7.8. Each 200 mg vial delivers 200 mg of coagulation factor Xa formulated with the inactive ingredients tromethamine (Tris base), Tris hydrochloride, L-arginine hydrochloride, sucrose (1% w/v), mannitol (2.5% w/v), and polysorbate 80 (0.01% w/v) at pH 7.8. 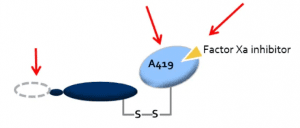 After reconstitution of the lyophilized powder with SWFI for IV administration, the product is a clear, colorless to slightly yellow solution. ANDEXXA contains no preservatives. The active ingredient in ANDEXXA is a genetically modified variant of human FXa. The active site serine was substituted with alanine, rendering the molecule unable to cleave and activate prothrombin. The gamma-carboxyglutamic acid (Gla) domain was removed to eliminate the protein's ability to assemble into the prothrombinase complex, thus removing the potential anti-coagulant effects. No additives of human or animal origin are used in the manufacture of ANDEXXA. The recombinant protein is produced in a genetically engineered Chinese Hamster Ovary (CHO) cell expression system and has a molecular weight of approximately 41 kDa. The manufacturing process incorporates two validated virus clearance steps. |
|||||||||||||||||||||||||||||||||||
CLINICAL PHARMACOLOGY OF ANDEXXA:
|
|||||||||||||||||||||||||||||||||||
| Mechanism of Action:
Coagulation factor Xa (recombinant), inactivated-zhzo exerts its procoagulant effect by binding and sequestering the FXa inhibitors, rivaroxaban and apixaban. Another observed procoagulant effect of the ANDEXXA protein is its ability to bind to, and inhibit the activity of Tissue Factor Pathway Inhibitor (TFPI). Inhibition of TFPI activity can increase tissue factor (TF)-initiated thrombin generation. |
|||||||||||||||||||||||||||||||||||
INDICATIONS AND USAGE
|
|||||||||||||||||||||||||||||||||||
| INDICATIONS AND USAGE:
ANDEXXA is indicated for patients treated with rivaroxaban or apixaban, when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding. This indication is approved under accelerated approval based on the change from baseline in anti-FXa activity in healthy volunteers. An improvement in hemostasis has not been established. Continued approval for this indication may be contingent upon the results of studies that demonstrate an improvement in hemostasis in patients. Limitations of Use |
|||||||||||||||||||||||||||||||||||
CONTRAINDICATIONS
|
|||||||||||||||||||||||||||||||||||
| Contraindications:
None. |
|||||||||||||||||||||||||||||||||||
PRECAUTIONS
|
|||||||||||||||||||||||||||||||||||
| WARNINGS AND PRECAUTIONS:
5.1 Thromboembolic and Ischemic Risks The thromboembolic and ischemic risks were assessed in 185 patients who received the Generation 1 product and in 124 patients who received the Generation 2 product. The median time to first event was six days, and patients were observed for these events for 30 days following the ANDEXXA infusion. Of the 86 patients who received the Generation 1 product and were re-anticoagulated prior to a thrombotic event, 11 (12.7%) patients experienced a thromboembolic event, ischemic event, cardiac event, or death. Monitor patients treated with ANDEXXA for signs and symptoms of arterial and venous thromboembolic events, ischemic events, and cardiac arrest. To reduce thromboembolic risk, resume anticoagulant therapy as soon as medically appropriate following treatment with ANDEXXA. The safety of ANDEXXA has not been evaluated in patients who experienced thromboembolic events or disseminated intravascular coagulation within two weeks prior to the life-threatening bleeding event requiring treatment with ANDEXXA. Safety of ANDEXXA also has not been evaluated in patients who received prothrombin complex concentrates, recombinant factor VIIa, or whole blood products within seven days prior to the bleeding event. 5.2 Re-elevation or Incomplete Reversal of Anti-FXa Activity The time course of anti-FXa activity following ANDEXXA administration was consistent among the healthy volunteer studies and the ANNEXA-4 study in bleeding patients [see Clinical Studies (14)]. Compared to baseline, there was a rapid and substantial decrease in anti-FXa activity corresponding to the ANDEXXA bolus. This decrease was sustained through the end of the ANDEXXA continuous infusion. The anti-FXa activity returned to the placebo levels approximately two hours after completion of a bolus or continuous infusion. Subsequently, the anti-FXa activity decreased at a rate similar to the clearance of the FXa inhibitors. Thirty-eight patients who received the Generation 1 product were anticoagulated with apixaban and had baseline levels of anti-FXa activity > 150 ng/mL. Nineteen of these 38 (50%) patients experienced a > 93% decrease from baseline anti-FXa activity after administration of ANDEXXA. Eleven patients who were anticoagulated with rivaroxaban had baseline anti-FXa activity levels > 300 ng/mL. Five of the 11 patients experienced a > 90% decrease from baseline anti-FXa activity after administration of ANDEXXA. Anti-FXa activity levels for patients who received the Generation 2 product were not available.
|
|||||||||||||||||||||||||||||||||||
ADVERSE REACTIONS ASSOCIATED with ANDEXXA
|
|||||||||||||||||||||||||||||||||||
| ADVERSE REACTIONS:
See PACKAGE INSERT for PATIENT COUNSELING INFORMATION and Medication Guide. Drug information (pdf) |
|||||||||||||||||||||||||||||||||||
DOSAGE AND ADMINISTRATION
|
|||||||||||||||||||||||||||||||||||
| DOSAGE AND ADMINISTRATION: For intravenous (IV) use only. Drug information (pdf)2.1 DoseThere are two dosing regimens (see Table 1). The safety and efficacy of an additional dose have not been established.=========================================== Table 1: ANDEXXA Dosing Regimens
The recommended dosing of ANDEXXA is based on the specific FXa inhibitor, dose of FXa inhibitor, and time since the patient's last dose of FXa inhibitor (see Table 2). =========================================== Table 2: ANDEXXA Dose Based on Rivaroxaban or Apixaban Dose (Timing of Last Dose of FXa Inhibitor before ANDEXXA Initiation)
=========================================== 2.2 Reconstitution
IV Bolus Preparation Use a 20-mL (or larger) syringe and 20-gauge (or higher) needle. Slowly inject the SWFI, directing the solution onto the inside wall of the vial to minimize foaming. To reduce the total reconstitution time needed during preparation, reconstitute all required vials in succession. To ensure dissolution of the cake or powder, gently swirl each vial until complete dissolution of powder occurs. Do not shake; shaking could lead to foaming. Typical dissolution time for each vial is approximately three to five minutes. If dissolution is incomplete, discard the vial, and do not use the product. Upon reconstitution, the parenteral drug product should be inspected visually for particulate matter and discoloration prior to administration. Use 60-mL (or larger) syringe with a 20-gauge (or higher) needle to withdraw the reconstituted ANDEXXA solution from each of the vials until the required dosing volume is achieved. Note the total volume withdrawn into the syringe. Transfer the ANDEXXA solution from the syringe into an empty polyolefin or polyvinyl chloride IV bag with a volume of 250 mL or less. Discard the vials, including any unused portion. Continuous IV Infusion Preparation
2.3 Administration
2.4 Restarting Antithrombotic Therapy
|
|||||||||||||||||||||||||||||||||||
HOW ANDEXXA IS SUPPLIED
|
|||||||||||||||||||||||||||||||||||
| DOSAGE FORMS AND STRENGTHS:
ANDEXXA is available as a white to off-white lyophilized powder in single-use vials of 100 mg or 200 mg of coagulation factor Xa (recombinant), inactivated-zhzo. |
|||||||||||||||||||||||||||||||||||
Storage and Stability
|
|||||||||||||||||||||||||||||||||||
| ANDEXXA is available as a white to off-white lyophilized powder in single-use vials of 100 mg or 200 mg of coagulation factor Xa (recombinant), inactivated-zhzo.
4 single use vials in a carton, each vial containing 100 mg of ANDEXXA 4 single use vials in a carton, each vial containing 200 mg of ANDEXXA Storage and Handling |
|||||||||||||||||||||||||||||||||||

